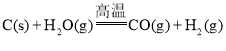��Ŀ����
����Ŀ����I����֪��448��ʱ����ӦH2(g)��I2(g)![]() 2HI(g)��ƽ�ⳣ��K1Ϊ49������¶��·�Ӧ
2HI(g)��ƽ�ⳣ��K1Ϊ49������¶��·�Ӧ![]() H2(g)��
H2(g)��![]() I2(g)
I2(g)![]() HI(g)��ƽ�ⳣ��K2Ϊ____________��
HI(g)��ƽ�ⳣ��K2Ϊ____________��
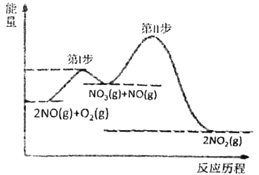
��II����һ��������ܱ������н������»�ѧ��Ӧ��CO2(g)��H2(g)![]() CO(g)��H2O(g)���仯ѧƽ�ⳣ��(K)���¶�(t)�Ĺ�ϵ�����ʾ��
CO(g)��H2O(g)���仯ѧƽ�ⳣ��(K)���¶�(t)�Ĺ�ϵ�����ʾ��
t/�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK��________��
��2���÷�ӦΪ________(����ȡ����ȡ�)��Ӧ��
��3�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������________��
A.������ѹǿ���� B.���������c(CO)����
C.v��(H2)��v��(H2O) D.c(CO2)��c(CO)
��4��ij�¶��£�ƽ��Ũ�ȷ�����ʽ��c(CO2)��c(H2)��c(CO)��c(H2O)�����жϴ�ʱ���¶�Ϊ________�档
��5����800��ʱ������������Ӧ��ijʱ�̲�������ڸ����ʵ�Ũ�ȷֱ�Ϊc(CO2)Ϊ2mol��L��1��c(H2)Ϊ1.5mol��L��1��c(CO)Ϊ1mol��L��1��c(H2O)Ϊ3mol��L��1������һʱ�̣���Ӧ��________(���������)���С�
���𰸡�7 ![]() ���� BC 830 ����
���� BC 830 ����
��������
����ѧ������Ϊ������ϵʱƽ�ⳣ��Ϊƽ����ϵ��
����ƽ�ⳣ��Ϊ������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ������¶����ߣ�ƽ�ⳣ��������ж���ЧӦ������ƽ������������������������ж��Ƿ��ƽ��״̬������Ũ�Ⱥ�ƽ�ⳣ��������ƽ�ⳣ�����Ӷ�ȷ���¶ȣ�����800��ʱ��Qc��K�Ƚ��жϷ�Ӧ�ķ���
��I����448��ʱ����ӦH2(g)+I2(g)2HI(g)��ƽ�ⳣ��K1Ϊ49����Ӧ![]() H2(g)��
H2(g)��![]() I2(g)
I2(g)![]() HI(g)��ƽ�ⳣ��K2Ϊ
HI(g)��ƽ�ⳣ��K2Ϊ![]() ���ʴ�Ϊ��7��
���ʴ�Ϊ��7��
��II����1��ƽ�ⳣ��Ϊ������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ���CO2(g)+H2(g)CO(g)+H2O(g)��ƽ�ⳣ��K=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��2�����¶����ߣ�ƽ�ⳣ������֪�������¶ȣ�ƽ��������Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
��3��A���÷�ӦΪ��������ʵ�������ķ�Ӧ����������ѹǿʼ�ղ��䣬������Ϊ�ж�ƽ��ķ�������A����
B�����������c(CO)���䣬��ﵽ��ѧƽ�⣬��B��ȷ��
C��v(H2)��=v(H2O)���������������˵���淴Ӧ������ȣ���ﵽƽ�⣬��C��ȷ��
D��c(CO2)=c(CO)���÷�Ӧ��һ���ﵽƽ�⣬Ũ�ȹ�ϵȡ���ڷ�Ӧ�����ʼ����ת���ʣ���D���ʴ�Ϊ��BC��
��4��c(CO2)c(H2)=c(CO)c(H2O)ʱ��ƽ�ⳣ��K=1������¶�Ϊ830�棬�ʴ�Ϊ��830��
��5����800��ʱ��K=0.9,Qc=![]() =
=![]() =1>K�����Ի�ѧ��Ӧ���淴Ӧ������У��ʴ�Ϊ������
=1>K�����Ի�ѧ��Ӧ���淴Ӧ������У��ʴ�Ϊ������

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��25���£���������ĵ��볣������������й�˵������ȷ����
���� | HCOOH | HClO | H2S |
����ƽ�ⳣ��(Ka) | Ka=1.0��10-4 | Ka=2.0��10-8 | Ka1=1.3��10-7 Ka2=7.1��10-15 |
A. �� HCOONa�� HCOOH�Ļ����Һ��pH=3����c(HCOOH)/c(HCOO-)=10
B. ��ͬŨ�ȵ� HCOONa�� NaClO��Һ��������Ũ��ǰ�ߴ�
C. �κ�Ũ��NaHS��Һ���ܴ��ڣ�c(H2S)+c(H+)=c(OH��)+c(S2��)
D. �� NaClO��Һ��ͨ��H2S�����ķ�ӦΪ2C1O��+H2S===S2��+2HClO