��Ŀ����
�������п�ͼ�ش����⣺

��֪��������A������к��������ᾧˮ��A��B������ˮ��G��һ����Ҫ����ԭ�ϣ���ͼ�в��ַ�Ӧ�������������ȥ����
��1��д������A�Ļ�ѧʽ��
 ��
��
��2��д��C��D+E��Ӧ�Ļ�ѧ����ʽ��
��3��д������Iͨ��H����Һ�з�Ӧ�����ӷ���ʽ��
��4��ijͬѧȡH����Һ��ͨ������D��������Һ����ֻ��һ�������ӣ���д�������仯�����е����ӷ���ʽ��

��֪��������A������к��������ᾧˮ��A��B������ˮ��G��һ����Ҫ����ԭ�ϣ���ͼ�в��ַ�Ӧ�������������ȥ����
��1��д������A�Ļ�ѧʽ��
��NH4��2Fe��SO4��2?6H2O
��NH4��2Fe��SO4��2?6H2O
�� ����B�ĵ���ʽ��

��2��д��C��D+E��Ӧ�Ļ�ѧ����ʽ��
4FeS+7O2
2Fe2O3+4SO2
| ||
4FeS+7O2
2Fe2O3+4SO2
��
| ||
��3��д������Iͨ��H����Һ�з�Ӧ�����ӷ���ʽ��
Fe3++3NH3?H2O=Fe��OH��3��+3NH4+
Fe3++3NH3?H2O=Fe��OH��3��+3NH4+
����4��ijͬѧȡH����Һ��ͨ������D��������Һ����ֻ��һ�������ӣ���д�������仯�����е����ӷ���ʽ��
2Fe3++SO2+2H2O=2Fe2++SO42-+4H+
2Fe3++SO2+2H2O=2Fe2++SO42-+4H+
����������ת����ϵ��֪����ɫ����L���������ᣬLΪBaSO4����Һ2����ɫΪ��ɫ����K+�����ɫ����ΪFe��OH��3��������IΪ������H�к�Fe3+����C��D��D��F��ת����֪����ɫ����CΪFeS2��DΪSO2��EΪFe2O3��FΪSO3��GΪ���ᣬ����HΪ����������ԭ���غ��֪����Һ1�к���������ӣ�������A������к��������ᾧˮ��A��B������ˮ����AΪ��NH4��2Fe��SO4��2?6H2O��BΪK2S��Ȼ����Ԫ�ػ�����֪ʶ����ѧ���������
����⣺��ת����ϵ��֪����ɫ����L���������ᣬLΪBaSO4����Һ2����ɫΪ��ɫ����K+�����ɫ����ΪFe��OH��3��������IΪ������H�к�Fe3+����C��D��D��F��ת����֪����ɫ����CΪFeS2��DΪSO2��EΪFe2O3��FΪSO3��GΪ���ᣬ����HΪ����������ԭ���غ��֪����Һ1�к���������ӣ�������A������к��������ᾧˮ��A��B������ˮ����AΪ��NH4��2Fe��SO4��2?6H2O��BΪK2S��
��1��������������֪AΪ��NH4��2Fe��SO4��2?6H2O��BΪK2S�������ʽΪ ���ʴ�Ϊ����NH4��2Fe��SO4��2?6H2O��
���ʴ�Ϊ����NH4��2Fe��SO4��2?6H2O�� ��
��
��2��C��D+E��Ӧ�Ļ�ѧ����ʽΪ4FeS+7O2
2Fe2O3+4SO2���ʴ�Ϊ��4FeS+7O2
2Fe2O3+4SO2��
��3��Iͨ��H����Һ�з�Ӧ�����ӷ���ʽΪFe3++3NH3?H2O=Fe��OH��3��+3NH4+���ʴ�Ϊ��Fe3++3NH3?H2O=Fe��OH��3��+3NH4+��
��4��H����Һ��ͨ������D��������Һ����ֻ��һ�������ӣ������ӷ�ӦΪ2Fe3++SO2+2H2O=2Fe2++SO42-+4H+���ʴ�Ϊ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
��1��������������֪AΪ��NH4��2Fe��SO4��2?6H2O��BΪK2S�������ʽΪ
 ���ʴ�Ϊ����NH4��2Fe��SO4��2?6H2O��
���ʴ�Ϊ����NH4��2Fe��SO4��2?6H2O�� ��
����2��C��D+E��Ӧ�Ļ�ѧ����ʽΪ4FeS+7O2
| ||
| ||
��3��Iͨ��H����Һ�з�Ӧ�����ӷ���ʽΪFe3++3NH3?H2O=Fe��OH��3��+3NH4+���ʴ�Ϊ��Fe3++3NH3?H2O=Fe��OH��3��+3NH4+��
��4��H����Һ��ͨ������D��������Һ����ֻ��һ�������ӣ������ӷ�ӦΪ2Fe3++SO2+2H2O=2Fe2++SO42-+4H+���ʴ�Ϊ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
���������⿼��������ƶϣ���������ɫ����ɫ��ӦΪ������ͻ�ƿڣ�ע������Ԫ�ء�ԭ���غ�ķ������Ƴ������ʣ���Ϥ������ԭ��Ӧ���ɽ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
�����Ŀ

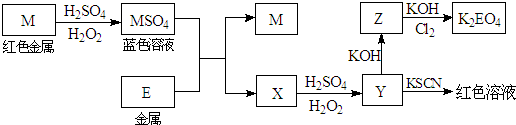
 ��2008?���գ��������п�ͼ�ش����⣨����ʱ������ʽ�е�M��E������Ӧ��Ԫ�ط��ű�ʾ����
��2008?���գ��������п�ͼ�ش����⣨����ʱ������ʽ�е�M��E������Ӧ��Ԫ�ط��ű�ʾ����
 ���˴Ź����ܹ����������ֽṹ���ں˴Ź��������У���ȷ�Ľṹ��
���˴Ź����ܹ����������ֽṹ���ں˴Ź��������У���ȷ�Ľṹ��