��Ŀ����
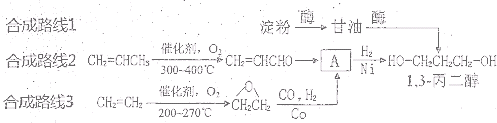
1��3-���������������߷��Ӳ���PTT����Ҫԭ�ϣ�Ŀǰ������·�������¼��֣�
[·��1]
[·��2]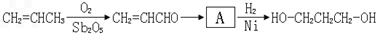
[·��3]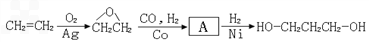

[·��2]
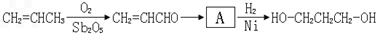
[·��3]

��1���л���A���еĹ�����Ϊ____________��
��2���Ӻϳ�ԭ����Դ�ĽǶȿ�������Ϊ����з�չǰ����·���� _______����1��2��3���������� ____________��
��3����1��3-��������Ա������ᣨ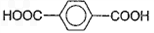 ��Ϊԭ�Ͽ��Ժϳɾ���PTT��д���仯ѧ����ʽ ______________��
��Ϊԭ�Ͽ��Ժϳɾ���PTT��д���仯ѧ����ʽ ______________��
��4����֪������������ܷ������·�Ӧ��
��2���Ӻϳ�ԭ����Դ�ĽǶȿ�������Ϊ����з�չǰ����·���� _______����1��2��3���������� ____________��
��3����1��3-��������Ա������ᣨ
 ��Ϊԭ�Ͽ��Ժϳɾ���PTT��д���仯ѧ����ʽ ______________��
��Ϊԭ�Ͽ��Ժϳɾ���PTT��д���仯ѧ����ʽ ______________����4����֪������������ܷ������·�Ӧ��
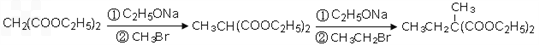
�Ա������������1,3-���������Ҵ�Ϊԭ�ϣ�������ѡ���ϳ�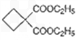 ����ת��Ϊ
����ת��Ϊ 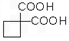 ��
��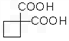 ��ͬ���칹�岻��������__________��
��ͬ���칹�岻��������__________��
a���� b���� c��ȩ d����
��5��Ҫ�ϳ� �������Ⱥϳ���Щ���ʣ����úϳɸ����ʵĻ�ѧ����ʽ�ش� __________��
�������Ⱥϳ���Щ���ʣ����úϳɸ����ʵĻ�ѧ����ʽ�ش� __________��
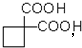
��6��������B��E��ת����ϵ����ͼ��ʾ����������������Cu(OH)2����Һ��1 mol B��Ӧ������1 mol Cu2O ��1 mol C��д����������������D��ͬ���칹��Ľṹ��ʽ��
�ٺ����������� �������Ҵ�����������Ӧ�� ��-OH��-Br����ͬһ��̼ԭ���ϡ�
________________________________________________________________
 ����ת��Ϊ
����ת��Ϊ  ��
�� ��ͬ���칹�岻��������__________��
��ͬ���칹�岻��������__________�� a���� b���� c��ȩ d����
��5��Ҫ�ϳ�
 �������Ⱥϳ���Щ���ʣ����úϳɸ����ʵĻ�ѧ����ʽ�ش� __________��
�������Ⱥϳ���Щ���ʣ����úϳɸ����ʵĻ�ѧ����ʽ�ش� __________����6��������B��E��ת����ϵ����ͼ��ʾ����������������Cu(OH)2����Һ��1 mol B��Ӧ������1 mol Cu2O ��1 mol C��д����������������D��ͬ���칹��Ľṹ��ʽ��
�ٺ����������� �������Ҵ�����������Ӧ�� ��-OH��-Br����ͬһ��̼ԭ���ϡ�
________________________________________________________________

��1���л���A���еĹ�����Ϊ��OH����CHO
��2��1��������·��1�Կ�������Դ����Ϊԭ�ϣ�·��2��3��ԭ��Ϊʯ�Ͳ�Ʒ����ʯ���Dz���������Դ
��3��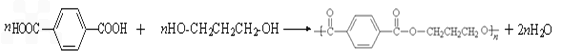
��4��b
��5��HO-CH2CH2CH2-OH + 2HBr ��BrCH2CH2CH2Br + 2H2O��
2CH3CH2OH + 2Na �� 2CH3CH2ONa + H2��
��6��2
��2��1��������·��1�Կ�������Դ����Ϊԭ�ϣ�·��2��3��ԭ��Ϊʯ�Ͳ�Ʒ����ʯ���Dz���������Դ
��3��
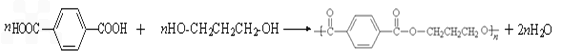
��4��b
��5��HO-CH2CH2CH2-OH + 2HBr ��BrCH2CH2CH2Br + 2H2O��
2CH3CH2OH + 2Na �� 2CH3CH2ONa + H2��
��6��2

��ϰ��ϵ�д�
 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
�����Ŀ





 ������Ƴ������ķ�Ӧ����ͼ
������Ƴ������ķ�Ӧ����ͼ

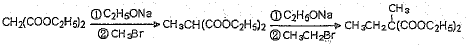
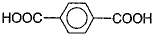 ��Ϊԭ�Ͽ��Ժϳɾ���PTT��д���仯ѧ����ʽ
��Ϊԭ�Ͽ��Ժϳɾ���PTT��д���仯ѧ����ʽ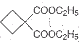 ����ת��Ϊ��
����ת��Ϊ��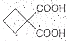 ��ͬ���칹�岻��������
��ͬ���칹�岻��������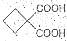 Ҫ�ϳɣ������Ⱥϳ���Щ���ʣ����úϳɸ����ʵĻ�ѧ����ʽ�ش�
Ҫ�ϳɣ������Ⱥϳ���Щ���ʣ����úϳɸ����ʵĻ�ѧ����ʽ�ش�

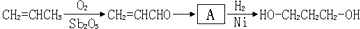

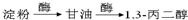

 ������Ƴ������ķ�Ӧ����ͼ______
������Ƴ������ķ�Ӧ����ͼ______