��Ŀ����
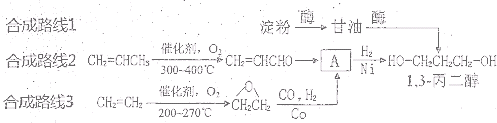
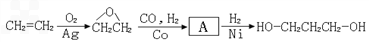
1��3-���������������߷��Ӳ���PTT����Ҫԭ�ϣ�Ŀǰ1��3-������������·���У���ʯ���ѽ���Ϊԭ�ϵ�����ʯ���ϳ�·�ߺ�һ�����﹤�̷��ϳ�·�ߡ���·��1����ϩȩˮ���⻯����![]()
��·��2�������������������
��·��3������ͷ�������![]() ����
����![]() 1��3-������
1��3-������
(1)A�Ľṹ��ʽΪ_______________________________________________________��
(2)�Ӻϳ�ԭ����Դ�ĽǶȿ�������Ϊ����з�չǰ����·����_____________(�1����2����3��)��������_______________________________________________________��
(3)��1��3-��������Ա�������Ϊԭ�Ͽ��Ժϳɾ���PTT��д���仯ѧ����ʽ��_______��
(4)��֪������������ܷ������·�Ӧ��
CH2(COOC2H5)2![]() CH3CH(COOC2H5)2
CH3CH(COOC2H5)2
���ø÷�Ӧԭ�����Ա������������1��3-���������Ҵ�Ϊԭ�Ϻϳ�![]() ��������Ƴ������ķ�Ӧ����ͼ����ʾ���ٺϳɹ��������Լ���ѡ���ںϳɷ�Ӧ����ͼ��ʾ����ʾ�����£�
��������Ƴ������ķ�Ӧ����ͼ����ʾ���ٺϳɹ��������Լ���ѡ���ںϳɷ�Ӧ����ͼ��ʾ����ʾ�����£�
A![]() B
B![]() C����
C����
(1)CH2OHCH2CHO
(2)3 ·��3�Կ�������Դ����Ϊԭ�ϣ�·��1��2��ԭ��Ϊʯ�Ͳ�Ʒ����ʯ���Dz���������Դ

(4)C2H5OH![]() C2H5ONa
C2H5ONa
HO��CH2CH2CH2��OH![]() CH2BrCH2CH2Br
CH2BrCH2CH2Br

������(1)A��![]()
(2)·��3�Կ�������Դ����Ϊԭ�ϣ�·��1��2��ԭ��Ϊʯ�Ͳ�Ʒ����ʯ���Dz���������Դ������·��3������з�չǰ����·�ߡ�
(4)C2H5OH![]() C2H5ONa��
C2H5ONa��







 ������Ƴ������ķ�Ӧ����ͼ
������Ƴ������ķ�Ӧ����ͼ

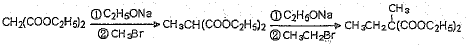
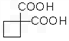
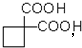
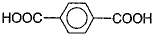 ��Ϊԭ�Ͽ��Ժϳɾ���PTT��д���仯ѧ����ʽ
��Ϊԭ�Ͽ��Ժϳɾ���PTT��д���仯ѧ����ʽ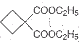 ����ת��Ϊ��
����ת��Ϊ��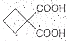 ��ͬ���칹�岻��������
��ͬ���칹�岻��������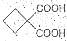 Ҫ�ϳɣ������Ⱥϳ���Щ���ʣ����úϳɸ����ʵĻ�ѧ����ʽ�ش�
Ҫ�ϳɣ������Ⱥϳ���Щ���ʣ����úϳɸ����ʵĻ�ѧ����ʽ�ش�
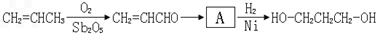
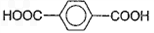 ��Ϊԭ�Ͽ��Ժϳɾ���PTT��д���仯ѧ����ʽ ______________��
��Ϊԭ�Ͽ��Ժϳɾ���PTT��д���仯ѧ����ʽ ______________��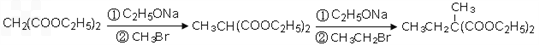
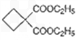 ����ת��Ϊ
����ת��Ϊ 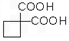 ��
�� ��ͬ���칹�岻��������__________��
��ͬ���칹�岻��������__________��  �������Ⱥϳ���Щ���ʣ����úϳɸ����ʵĻ�ѧ����ʽ�ش� __________��
�������Ⱥϳ���Щ���ʣ����úϳɸ����ʵĻ�ѧ����ʽ�ش� __________��
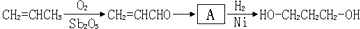

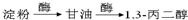

 ������Ƴ������ķ�Ӧ����ͼ______
������Ƴ������ķ�Ӧ����ͼ______