��Ŀ����
4��ʵ�������ú�����CO2���ʵ�CO����ԭ����������FexOy��֤��CO�ܹ���ԭFexOy���ұ���������ΪCO2��ʵ�����ṩ�ĸ���������ҩƷ���£�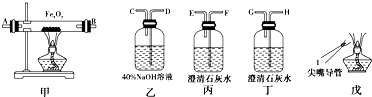
��1��ʵ��ʱ����������װ�õ���ȷ����˳����д���ӿڵĴ��ţ�����������D��C�ӣ�F������E����A��B��H��G��I��
��2����װ�����з�����Ӧ�Ļ�ѧ����ʽ��2NaOH+CO2=Na2CO3+H2O��
��3����װ�ü��з�����Ӧ�Ļ�ѧ����ʽ��FexOy+yCO$\frac{\underline{\;����\;}}{\;}$xFe+yCO2��
��4����װ���г���ʯ��ˮ�������Ǽ���CO�е�CO2�Ƿ�����
��5��ʵ������У��ܹ�˵��CO��������CO2��ʵ�������Ƕ��г����ʯ��ˮ����ǣ�
���� ��1����ʵ��Ŀ�ġ��ú�����CO2���ʵ�CO����ԭ����������FexOy����֤��CO�ܹ���ԭFexOy���ұ���������ΪCO2������ÿ��װ�õ����ã�Ȼ�����Ӹ�װ�ã�
��2��װ������ʢ�ŵ���NaOH��Һ���ܹ��������̼��Ӧ����̼���ƣ����ڳ�ȥ��������еĶ�����̼��
��3��װ�ü���CO��FexOy�ڸ����·�Ӧ���ɶ�����̼�������ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��4����ʵ��Ŀ������Ҫ����CO�������������̼����ԭ��������еĶ�����̼�����ȥ������г���ʯ��ˮ����֤��������̼�Ѿ�������
��5����������Ϊ����ɫ����������������Ӷ�����ɫ��Ϊ��ɫʱ��֤����������CO��ԭ�������г���ʯ��ˮ�����ʱ��֤��CO�������ɶ�����̼��
��� �⣺��1��ʵ�������ú�����CO2���ʵ�CO����ԭ����������FexOy����֤��CO�ܹ���ԭFexOy���ұ���������ΪCO2��������Ҫ������������Һ��ȥ��������еĶ�����̼��Ȼ���ó���ʯ��ˮ���������̼�Ѿ���������ʼ��CO��ԭ��������������ó���ʯ��ˮ����CO�������ɶ�����̼������õ�ȼ�ķ�����ȥʣ���CO������װ������˳��Ϊ����������D��C�ӣ�F������E����A��B��H��G��I��
�ʴ�Ϊ��D��C��A��B��H��G��I��
��2��װ�����е�����������Һ�ܹ��������̼��Ӧ����̼���ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��2NaOH+CO2=Na2CO3+H2O��
�ʴ�Ϊ��2NaOH+CO2=Na2CO3+H2O��
��3��װ�ü���FexOy��һ����̼�ڸ����·���������ԭ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ��FexOy+yCO$\frac{\underline{\;����\;}}{\;}$xFe+yCO2��
�ʴ�Ϊ��FexOy+yCO$\frac{\underline{\;����\;}}{\;}$xFe+yCO2��
��4�����г���ʯ��ˮ�ܹ�����ԭ��������еĶ�����̼�Ѿ��������Ӷ������˶Ժ���ʵ��ĸ��ţ�
�ʴ�Ϊ������CO�е�CO2�Ƿ�����
��5����������Ϊ����ɫ��Fe2O3��������Ӷ�����ɫ��Ϊ��ɫʱ��֤����������CO��ԭ�������г���ʯ��ˮ�����ʱ��֤��CO�������ɶ�����̼��
�ʴ�Ϊ�����г����ʯ��ˮ����ǣ�
���� ���⿼��ʵ��װ�õ��ۺ�Ӧ�ü������仯��������ʵ�飬��Ŀ�Ѷ��еȣ�����֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ���ѧ���ķ�����������������ѧʵ��������ע�����������仯�������ʣ���ȷʵ��Ŀ��Ϊ���ؼ���ע�⻯ѧʵ���������������ʵ��װ�õ�����˳��

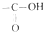 �е�-OH��±ԭ��ȡ�����õĻ������Ϊ��±�����л������п��Կ�����±���ǣ�������
�е�-OH��±ԭ��ȡ�����õĻ������Ϊ��±�����л������п��Կ�����±���ǣ�������| A�� | CCl4 | B�� | CH3COF | C�� | CH3COCH2Cl | D�� | CH2ClCOOH |
| A�� | 1-�����������Ļ������ͨ�����ȵ�ͭ�����ɱ�ȩ | |
| B�� | 1-�������ܺ���±�ᷢ����Ӧ | |
| C�� | 1-�����ķе�����Ҵ� | |
| D�� | 1-������2-���������ѻ�Ϊͬ���칹�� |
| A�� | �ù��˵ķ�����ȥʳ��ˮ�е���ɳ | B�� | ������ķ���������ˮ�Ƴ�����ˮ | ||
| C�� | �������ȥ�������е������� | D�� | ���ȳ�ȥNa2CO3�����е�NaHCO3 |
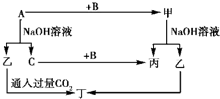 A��B��C�ǵ��ʣ�����A�ǽ������������ʼ��ת����ϵ��ͼ��ʾ��
A��B��C�ǵ��ʣ�����A�ǽ������������ʼ��ת����ϵ��ͼ��ʾ�� ʯ���裬�����谱���ƣ�CaCN2���������ƺ��������������ʹ��ɵĻ����ʻҺ�ɫ���������ζ����һ�ּ��Է��ϣ�Ҳ�Ǹ�Ч�Ͷ������ũҩ����Ҫԭ��֮һ�����������ݼ���ɱ������ɱ����ȣ�����������˫�谷�������谷��������ȣ�
ʯ���裬�����谱���ƣ�CaCN2���������ƺ��������������ʹ��ɵĻ����ʻҺ�ɫ���������ζ����һ�ּ��Է��ϣ�Ҳ�Ǹ�Ч�Ͷ������ũҩ����Ҫԭ��֮һ�����������ݼ���ɱ������ɱ����ȣ�����������˫�谷�������谷��������ȣ�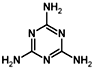 ���׳ơ���������Ҳ�����谱�����Ƶã��������谷�����к��еĦҼ��ͦм���Ŀ֮��Ϊ5��1��
���׳ơ���������Ҳ�����谱�����Ƶã��������谷�����к��еĦҼ��ͦм���Ŀ֮��Ϊ5��1��