��Ŀ����
16����Ҫ��д����ʽ����1��̼��ƺ����ᣨд�����ӷ���ʽ��CaCO3+2H+=Ca2++H2O+CO2��
��2������������Һ��ϡ���ᣨд�����ӷ���ʽ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O
��3��Fe2��SO4��3��д�����뷽��ʽ��Fe2��SO4��3=2Fe3++3SO42-
��4��H++OH-=H2O��д����Ӧ�Ļ�ѧ����ʽ��HCl+NaOH=NaCl+H2O
��5������������Na+��K+��Cu2+��H+��NO3-��Cl-��CO32-��OH-�����ܴ���������ͬһ��Һ������������Ƿֳ�A��B���飬����ÿ���о������������Ӻ����������ӣ�
A�飺Cu2+ ��H+Cl-NO3-
B�飺Na+K+OH-CO32-��
���� ��1��̼��ƺ����ᷴӦ�����Ȼ��ƺ�ˮ��������̼��
��2������������Һ��ϡ���ᷴӦ�������ᱵ��ˮ��
��3��������Ϊǿ����ʣ���ȫ���룻
��4��H++OH-=H2O��ʾǿ���������ǿ�Ӧ����ˮ�Ϳ������Σ�
��5���������ӷ�Ӧ�����жϣ��ܹ���Ӧ�����Ӳ��ܹ��森
��� �⣺��1��̼��ƺ����ᷴӦ�����Ȼ��ƺ�ˮ��������̼�����ӷ���ʽ��CaCO3+2H+=Ca2++H2O+CO2����
�ʴ�Ϊ��CaCO3+2H+=Ca2++H2O+CO2����
��2������������Һ��ϡ���ᷴӦ�������ᱵ��ˮ�����ӷ���ʽ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O��
�ʴ�Ϊ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O��
��3��������Ϊǿ����ʣ���ȫ��������������Ӻ���������ӣ����뷽��ʽ��Fe2��SO4��3=2Fe3++3SO42-��
�ʴ�Ϊ��Fe2��SO4��3=2Fe3++3SO42-��
��4��H++OH-=H2O���Ա�ʾ�������������Ʒ�Ӧ�����ӷ���ʽ����ѧ����ʽΪ��HCl+NaOH=NaCl+H2O��
�ʴ�Ϊ��HCl+NaOH=NaCl+H2O��
��5��A�к���ͭ���ӣ������������ӡ�̼��������ܹ���ͭ���ӷ�Ӧ���Զ���Ӧ��B���У�A������������ֻ���������Ӻ���������ӣ�
B�к���̼������ӡ����������ӣ���һ�����ܺ��������ӣ�����������ֻ����A���У�B������������Ϊ�����Ӻͼ����ӣ�
����A��������Cu2+ H+Cl-NO3-��B��������Na+K+OH-CO32-��
�ʴ�Ϊ��H+Cl-NO3-��Na+K+OH-CO32-��
���� ���⿼�������ӷ���ʽ��д�����ӹ����жϣ���ȷ���ӷ���ʽ��д�ķ�����ע�������ǽ���ؼ���ע�����ӹ����������жϣ���Ŀ�ѶȲ���

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�| A�� | �Ȼ��ƾ�������ˮ | B�� | ����ؾ�����ۻ�����ȴ | ||
| C�� | ����������ɱ������ | D�� | ������·��ˮ���� |
| A�� | ��ʹpH��ֽ�ʺ�ɫ����Һ��CH3COOH��NH4+��I-��NO3- | |
| B�� | pH=14����Һ��Ba2+��Mg2+��HCO3- | |
| C�� | c��Fe3+��=0.1mol/L����Һ��H+��Al3+��Cl-��SCN- | |
| D�� | kw/c��H+��=0.1mol/L����Һ��Na+��NH3•H2O��SiO32-��NO3- |
| A�� | ��״���£�2.24L�״��к��е�ԭ������Ϊ0.6NA | |
| B�� | �����£�500mL pH=1��ϡ�����к��е�H+����ԼΪ0.05NA | |
| C�� | 7.8g Na2O2�����к��е���������Ϊ0.4NA | |
| D�� | ��⾫��ͭʱ���������õ���������Ϊ2NA����������������64g |
| A�� | K+��Na+��CO32-��Cl- | B�� | Ca2+��K+��CO32-��OH- | ||
| C�� | Fe3+��Ba2+��NO3-��SO4- | D�� | H+��Na+��HCO3-��OH- |
| A�� | ������п�����·���������� | |
| B�� | п�Ǹ����������������� | |
| C�� | ����ʱ����������ҺpH��С��������pH���� | |
| D�� | ��Һ��OH-�������ƶ���K+��H+���ƶ� |

 ��G�Ľṹ��ʽ��
��G�Ľṹ��ʽ�� ��
�� +2Cu��OH��2$\stackrel{��}{��}$
+2Cu��OH��2$\stackrel{��}{��}$ +Cu2O��+2H2O��
+Cu2O��+2H2O�� +CH3OH$��_{��}^{Ũ����}$
+CH3OH$��_{��}^{Ũ����}$ +H2O��
+H2O��
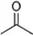 ���ݼ���ʽ�ش��������⣺
���ݼ���ʽ�ش��������⣺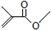 ����ʽ��C5H8O2��
����ʽ��C5H8O2��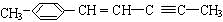 �����������4��ԭ�ӹ�ֱ�ߣ�
�����������4��ԭ�ӹ�ֱ�ߣ�
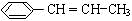 ���ڴ������������ɾ۱���ϩ�ķ�Ӧ����ʽ��
���ڴ������������ɾ۱���ϩ�ķ�Ӧ����ʽ��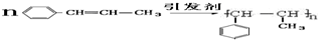 ��
��