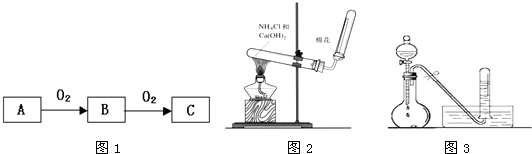
��Ŀ����
A��B��C����ѧ��ѧ�����ĵ��ʣ�����A�ǽ������ס��ҡ����������������ֻ������������ͼ��ת����ϵ�����ǹ�ҵ����ȡA����Ҫԭ�ϣ�

��1��д���������ʵĻ�ѧʽ��A
��2��д���������ڹ�ҵ�ϵ�����һ����Ҫ��;
��3��д�����б仯�ķ���ʽ��
��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
�ڹ�ҵ���ü��Ʊ�A�Ļ�ѧ����ʽ
���������CO2��Ӧ�����ӷ���ʽ

��1��д���������ʵĻ�ѧʽ��A
Al
Al
����AlCl3
AlCl3
����2��д���������ڹ�ҵ�ϵ�����һ����Ҫ��;
�����²��ϡ���ȡ������
�����²��ϡ���ȡ������
����3��д�����б仯�ķ���ʽ��
��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
2Al+2OH-+2H2O�T2AlO2-+3H2��
2Al+2OH-+2H2O�T2AlO2-+3H2��
�ڹ�ҵ���ü��Ʊ�A�Ļ�ѧ����ʽ
2Al2O3
4Al+3O2��
| ||
2Al2O3
4Al+3O2��
| ||
���������CO2��Ӧ�����ӷ���ʽ
CO2+AlO2-+2H2O�TAl��OH��3��+HCO3-
CO2+AlO2-+2H2O�TAl��OH��3��+HCO3-
��������AΪ�������ɵ���A��NaOH��Һ��Ӧ�����ɵ���C����CΪH2��A����������Ϊƫ�����ƣ�������Ķ�����̼��Ӧ���ɶ�Ϊ���������������ȷֽ�õ���Ϊ�����������ڼ������ᷴӦ���ʼ�Ϊ����������AΪAl��BΪO2����ΪNaAlO2����Ϊ�������������ת����ѡ��֪����Ϊˮ����ΪAlCl3��Ȼ�������ʵ����ʼ���ѧ���������
����⣺AΪ����������A��NaOH��Һ��Ӧ�����ɵ���C����CΪH2��A����������Ϊƫ�����ƣ�������Ķ�����̼��Ӧ���ɶ�Ϊ���������ᣬ�����ȷֽ�õ���Ϊ�����������ڼ������ᷴӦ���ʼ�Ϊ����������AΪAl��BΪO2����ΪNaAlO2����Ϊ�������������ת����ѡ��֪����Ϊˮ����ΪAlCl3��
��1��������������֪��AΪAl����ΪAlCl3���ʴ�Ϊ��Al��AlCl3��
��2���������������ڹ�ҵ�Ͽ��������²��ϡ���ȡ�����ʵȣ�
�ʴ�Ϊ�������²��ϡ���ȡ�����ʣ�
��3����Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O�T2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����
�ڢڹ�ҵ���ü��Ʊ�A�Ļ�ѧ����ʽΪ2Al2O3
4Al+3O2�����ʴ�Ϊ��2Al2O3
4Al+3O2����
���������CO2��Ӧ�����ӷ���ʽΪCO2+AlO2-+2H2O�TAl��OH��3��+HCO3-���ʴ�Ϊ��CO2+AlO2-+2H2O�TAl��OH��3��+HCO3-��
��1��������������֪��AΪAl����ΪAlCl3���ʴ�Ϊ��Al��AlCl3��
��2���������������ڹ�ҵ�Ͽ��������²��ϡ���ȡ�����ʵȣ�
�ʴ�Ϊ�������²��ϡ���ȡ�����ʣ�
��3����Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O�T2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����
�ڢڹ�ҵ���ü��Ʊ�A�Ļ�ѧ����ʽΪ2Al2O3
| ||
| ||
���������CO2��Ӧ�����ӷ���ʽΪCO2+AlO2-+2H2O�TAl��OH��3��+HCO3-���ʴ�Ϊ��CO2+AlO2-+2H2O�TAl��OH��3��+HCO3-��
���������⿼��������ƶϣ��漰�����ʼ��仯��������ʣ����������ʼ������������ǹؼ�������A��NaOH��Һ��Ӧ�ǡ�ͻ�ƿڡ����ѶȲ���

��ϰ��ϵ�д�
 �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�����Ŀ



 HS-+OH-
HS-+OH- Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������
Al��OH��3+3H+����ɫ����Ͱ�ɫ��״�������� ��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��
��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��