��Ŀ����
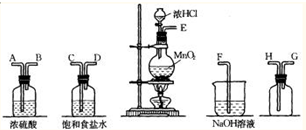
BrCl�е�Ϊ10�棬��ˮ����ˮ�⣬��ǿ�ҵ������ԣ��ǹ�ҵ����Ҫ��ˮ����ɱ������Br2�е�Ϊ58.8�森ijʵ��С����ʵ���Һϳ�BrCl����װ����ͼ��ʾ���г�������ȥ����

�ش��������⣺
��1��װ��C�е��Լ�Ϊ______��
��2��װ��A�з�����Ӧ�����ӷ���ʽΪ______��
��3��װ��D�����������������ܵ�������______��װ��D��ˮԡ���Ȳ������¶�40-42�棬�¶Ȳ��˹��ߵ�ԭ����______��
��4���ᴿE�еõ��Ĵ�BrCl��ʵ�����������______��

�ش��������⣺
��1��װ��C�е��Լ�Ϊ______��
��2��װ��A�з�����Ӧ�����ӷ���ʽΪ______��
��3��װ��D�����������������ܵ�������______��װ��D��ˮԡ���Ȳ������¶�40-42�棬�¶Ȳ��˹��ߵ�ԭ����______��
��4���ᴿE�еõ��Ĵ�BrCl��ʵ�����������______��
��1��BrCl�е�Ϊ10�棬��ˮ����ˮ�⣬��ǿ�ҵ������ԣ����ܺ����������壬����װ��ͼ��֪��Aװ���Ƿ�Ӧ���������ķ���װ�ã����ɵ������к���ˮ�������Ȼ��⣬ͨ��װ��B�б���ʳ��ˮ�����Ȼ��⣬����װ��C�е�Ũ��������ˮ������
�ʴ�Ϊ��Ũ���
��2��װ��A�����Ʊ������ķ�Ӧ�����ö������̺�Ũ������ȷ�Ӧ�����Ȼ��̡�������ˮ����Ӧ�����ӷ���ʽΪ��MnO2+4H++2Cl-
Mn2++Cl2��+2H2O��
�ʴ�Ϊ��MnO2+4H++2Cl-
Mn2++Cl2��+2H2O��
��3��װ��D�����������������ܵ�����������������װ��D��ˮԡ���Ȳ������¶�40-42�棬Br2�е�Ϊ58.8�棬Ŀ���DZ�����ӷ���
�ʴ�Ϊ�����������壬�¶ȹ��ᵼ�´�����ӷ���
��4��BrCl�е�Ϊ10�棬��������ķ����õ���BrCl���ʴ�Ϊ������
�ʴ�Ϊ��Ũ���
��2��װ��A�����Ʊ������ķ�Ӧ�����ö������̺�Ũ������ȷ�Ӧ�����Ȼ��̡�������ˮ����Ӧ�����ӷ���ʽΪ��MnO2+4H++2Cl-
| ||
�ʴ�Ϊ��MnO2+4H++2Cl-
| ||
��3��װ��D�����������������ܵ�����������������װ��D��ˮԡ���Ȳ������¶�40-42�棬Br2�е�Ϊ58.8�棬Ŀ���DZ�����ӷ���
�ʴ�Ϊ�����������壬�¶ȹ��ᵼ�´�����ӷ���
��4��BrCl�е�Ϊ10�棬��������ķ����õ���BrCl���ʴ�Ϊ������

��ϰ��ϵ�д�
�����Ŀ