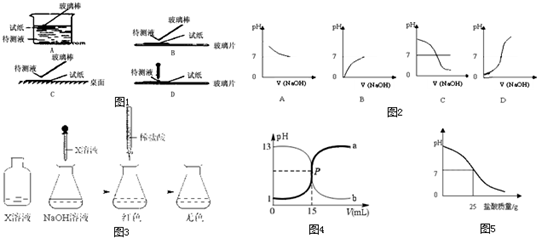
��Ŀ����
��1������ʵ������ѡ�õ�������������______
A����200mL��Ͳ��ȡ5.2mLϡH2SO4
B����100mL�ձ�����100g��������Ϊ1%��ʳ��ˮ
C����������ƽ��ȡ11.7gNaCl����
D���ü�ʽ�ζ�����ȡ25.1mL��ˮ
E����250mL����ƿ����250mL0.20mol/L������������Һ
��2������ʵ������Dz���ȷ�ģ��뽫��Щ�������ܷ����IJ���������ڿհ��У�
A�����Թ��е�Һ�����ʱ��Һ�峬���Թ��ݻ���
______��
B���ü�ʽ�ζ���ʢװKMnO4��Һ______��
��3������һ�����ʵ���Ũ�ȵ���Һʱ
�������������Ϊ______
��������ʱ���ӣ�������Һ�����ʵ���Ũ�ȱ�Ҫ���ֵ______���ƫ�ߡ���ƫ�͡���
�������ݺ�ҡ�Ⱦ��ã����ְ�Һ����ڿ̶��ߣ���ʱӦ�ã����ţ�______
A��ֱ��ת�Ƶ��Լ�ƿ��
B����ˮ���¶��ݺ�����������ƿ��
C�����¶��ݺ���ת�Ƶ��Լ�ƿ�У�
A����200mL��Ͳ��ȡ5.2mLϡH2SO4
B����100mL�ձ�����100g��������Ϊ1%��ʳ��ˮ
C����������ƽ��ȡ11.7gNaCl����
D���ü�ʽ�ζ�����ȡ25.1mL��ˮ
E����250mL����ƿ����250mL0.20mol/L������������Һ
��2������ʵ������Dz���ȷ�ģ��뽫��Щ�������ܷ����IJ���������ڿհ��У�
A�����Թ��е�Һ�����ʱ��Һ�峬���Թ��ݻ���
| 1 |
| 3 |
B���ü�ʽ�ζ���ʢװKMnO4��Һ______��
��3������һ�����ʵ���Ũ�ȵ���Һʱ
�������������Ϊ______
��������ʱ���ӣ�������Һ�����ʵ���Ũ�ȱ�Ҫ���ֵ______���ƫ�ߡ���ƫ�͡���
�������ݺ�ҡ�Ⱦ��ã����ְ�Һ����ڿ̶��ߣ���ʱӦ�ã����ţ�______
A��ֱ��ת�Ƶ��Լ�ƿ��
B����ˮ���¶��ݺ�����������ƿ��
C�����¶��ݺ���ת�Ƶ��Լ�ƿ�У�
��1��A����ȡ5.2mL��ϡ���ᣬѡ��200mL��Ͳ����Ӧѡ��10mL��Ͳ����A����
B��100mL�ձ����ܾ�ȷ��ȡ99�˵�ˮ����B����
C��������ƽ��ȷ��0.1g����������������ƽ��ȡ11.7gNaCl���壬��C��ȷ��
D����ˮ��ǿ�����ԣ��ḯʴ��ʽ�ζ��ܵ��ܣ���D����
E��250mL����ƿ������250mL0.2mol/L������������Һ����E��ȷ��
��ѡ��C��E��
��2�����Թ��ڵ�Һ�����ʱ��Һ�岻�ó����Թ��ݻ���
���������ʱ����������ˣ�
KMnO4��Һ��ǿ�����ԣ��ḯʴ��ʽ�ζ��ܵ��ܣ�
�ʴ�Ϊ��Һ�����ʱ�׳���Թܣ���ʽ�ζ��ܵ��ܿɱ���ʴ���ζ���©ˮ��
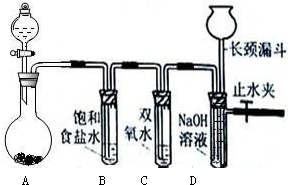
��3����ʵ������IJ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ��������Ͳ�ͽ�ͷ�ι���ȡҺ�壩�����ձ����ܽ��ϡ�ͣ����ò��������裬�ָ����º�ת�Ƶ�һ����������ƿ�У����ò�����������ϴ���ձ��Ͳ�����2��3�Σ�����ϴ��Һ��������ƿ�У���������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμ�ˮ����Һ����̶���ˮƽ���У��Ǻ�ƿ���������ߵ�����ҡ�ȣ������Լ�ƿ����ǩ���森
������Ҫ������Ϊ����Ͳ����������ƽ�����ձ���������������ƿ����ͷ�ιܣ�
�ʴ�Ϊ����Ͳ����������ƽ�����ձ���������������ƿ����ͷ�ιܣ�
�ڶ���ʱ���ӣ���Һ�������С������c=
����������Һ�����ʵ���Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�۶��ݺ�ҡ�Ⱦ��ã����ְ�Һ����ڿ̶��ߣ�����Ϊ��Һ�����ڿ̶������ϣ�����ҺŨ����Ӱ�죬����ֱ��ת�Ƶ��Լ�ƿ�У���ѡA��
B��100mL�ձ����ܾ�ȷ��ȡ99�˵�ˮ����B����
C��������ƽ��ȷ��0.1g����������������ƽ��ȡ11.7gNaCl���壬��C��ȷ��
D����ˮ��ǿ�����ԣ��ḯʴ��ʽ�ζ��ܵ��ܣ���D����
E��250mL����ƿ������250mL0.2mol/L������������Һ����E��ȷ��
��ѡ��C��E��
��2�����Թ��ڵ�Һ�����ʱ��Һ�岻�ó����Թ��ݻ���
| 1 |
| 3 |
KMnO4��Һ��ǿ�����ԣ��ḯʴ��ʽ�ζ��ܵ��ܣ�
�ʴ�Ϊ��Һ�����ʱ�׳���Թܣ���ʽ�ζ��ܵ��ܿɱ���ʴ���ζ���©ˮ��
��3����ʵ������IJ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ��������Ͳ�ͽ�ͷ�ι���ȡҺ�壩�����ձ����ܽ��ϡ�ͣ����ò��������裬�ָ����º�ת�Ƶ�һ����������ƿ�У����ò�����������ϴ���ձ��Ͳ�����2��3�Σ�����ϴ��Һ��������ƿ�У���������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμ�ˮ����Һ����̶���ˮƽ���У��Ǻ�ƿ���������ߵ�����ҡ�ȣ������Լ�ƿ����ǩ���森
������Ҫ������Ϊ����Ͳ����������ƽ�����ձ���������������ƿ����ͷ�ιܣ�
�ʴ�Ϊ����Ͳ����������ƽ�����ձ���������������ƿ����ͷ�ιܣ�
�ڶ���ʱ���ӣ���Һ�������С������c=
| n |
| v |
�ʴ�Ϊ��ƫ�ߣ�
�۶��ݺ�ҡ�Ⱦ��ã����ְ�Һ����ڿ̶��ߣ�����Ϊ��Һ�����ڿ̶������ϣ�����ҺŨ����Ӱ�죬����ֱ��ת�Ƶ��Լ�ƿ�У���ѡA��

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ