��Ŀ����
8���ȣ�Sr��Ϊ�������ڢ�A��Ԫ�أ��ߴ���ˮ�Ȼ��Ⱦ��壨SrCl2•6H2O�����кܸߵľ��ü�ֵ��61��ʱ���忪ʼʧȥ�ᾧˮ��100��ʱʧȥȫ���ᾧˮ���ù�ҵ̼���ȷ�ĩ��������Ba��Fe�Ļ�����Ʊ��ߴ���ˮ�Ȼ��ȵĹ�����ͼ��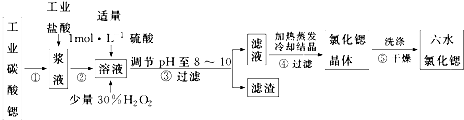
��ش𣺣�1��������е�����ҺpH��8��10����ѡ�õ��Լ�ΪBD��
A��ϡ���� B���������ȷ�ĩ C���������� D�������ȷ�ĩ
������������Ҫ�ɷ���BaSO4��Fe��OH��3���ѧʽ����
��2������Һ��Ba2+ Ũ��Ϊ1��10-5mol•L-1�������±����ݿ���������Һ��Sr2+���ʵ���Ũ��Ϊ������0.03mol/L��
| SrSO4 | BaSO4 | Sr��OH��2 | Fe��OH��3 | Fe��OH��2 | |
| Ksp | 3.3��10-7 | 1.1��10-10 | 3.2��10-4 | 2.6��10-39 | 4.87��10-17 |
���� ��1������pH��ȥFe3+�ȣ������������ʣ�����ǰ�������ϡ����������ᱵ���ɣ�
��2������BaSO4��Ksp�����Һ���������Ũ�ȣ��ٸ���SrSO4��Ksp���Sr2+���ʵ���Ũ�ȣ�
��� �ڣ���1������pH��ȥFe3+�ȣ�Ҫ�����������ʣ����ѡ���������ȷ�ĩ�������ȷ�ĩ������ǰ�������ϡ����������ᱵ���ɣ����Գ��������ּ�BaSO4��Fe��OH��3��
�ʴ�Ϊ��B D��BaSO4��Fe��OH��3��
��2����Һ��Ba2+Ũ��Ϊ1��10-5mol•L-1����c��SO42-��=$\frac{Ksp��BaS{O}_{4}��}{c��B{a}^{2+}��}$=$\frac{1.1��1{0}^{-10}}{1��1{0}^{-5}}$=1.1��10-5mol/L��
������Һ��Sr2+���ʵ���Ũ��Ϊc��Sr2+��=$\frac{Ksp��SrS{O}_{4}��}{c��S{{O}_{4}}^{2-}��}$=$\frac{3.3��1{0}^{-7}}{1.1��1{0}^{-5}}$=0.03mol/L����Sr2+���ʵ���Ũ�ȴ���0.03mol/L�ǻ�����SrSO4������������Һ��Sr2+���ʵ���Ũ�Ȳ�����0.03mol/L��
�ʴ�Ϊ��������0.03mol/L��
���� ���Ȼ��ȵ��Ʊ�Ϊ���壬����ѧ���Թ������̵����⡢���ʵķ����ᴿ������ת���ȣ���Ҫѧ���߱��Ķ������������������֪ʶ�Ľ��������������Ŀ�ѶȲ���

| A�� | �����������ᣬ�۲����� | B�� | �������ʯ��ˮ���۲����� | ||
| C�� | �����Ȼ�����Һ���۲����� | D�� | ͨ������CO2���壬�۲����� |
| A�� | ������Һ | B�� | Ũʳ�� | C�� | Ũ���� | D�� | ŨAlCl3��Һ |
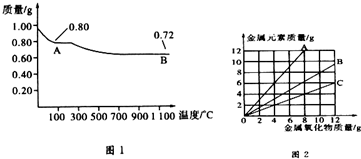
| A�� | ͼ1�в���A��B�Ļ�ѧʽ�ֱ�ΪCu2O��CuO | |
| B�� | ͼ1���������й�����0.26gˮ | |
| C�� | ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������C | |
| D�� | ͼ1�У�A��B��������0.01 mol���ӷ�����ת�� |
| A�� | ԭ����һ���IJ��ɷָ��ʵ������ | |
| B�� | ԭ�Ӻ��������������ں��������������ԭ���Ե��� | |
| C�� | ��ͬ�����ԭ�ӣ�������������ͬ�����������Ҳ��ͬ | |
| D�� | ��ͬ�����ԭ�ӣ�������������ͬ�����������������ͬ |
| A�� | ��25mL��ʽ�ζ�����ȡ18.40mL NaOH��Һ | |
| B�� | ��100mL ��Ͳ��ȡ5.20mL ���� | |
| C�� | ��������ƽ��ȡ25.20g NaCl���� | |
| D�� | ��100mL ����ƿ����125mL 0.1mol•L-1���� |
| ԭ������ | �����Ų�ʽ | �۲�����Ų� | ���� | �� |
| 17 | ��1s2 2s22p6 3s23p5 | ��3s23p5 | ��3 | �ܢ���A |
| ��10 | 1s22s22p6 | ��2s22p6 | ��2 | ��0 |
| ��24 | ��1s2 2s22p6 3s23p63d5 4s1 | 3d54s1 | ��11�� | ��B |
�ٸ������֡��۱���ϩ����֬�����������ȼ����������ǻ����
�ڱ��ɱ������������������ʱ�Ƿ��Ӿ���
��С�մ�Ӳ֬���ơ�BaSO4��Al2O3����ǿ�����
��CO2��NH3��SO2��H2S��Cl2���Ƿǵ����
�ݼ�������Һ��������Һ�������������ǽ���
�ޱ��ӣ����������H2O2�ֱ������ᡢ��κ������
| A�� | �٢ۢ� | B�� | �٢ڢܢ� | C�� | �٢ڢۢ� | D�� | �٢ڢۢܢݢ� |