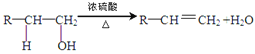
��Ŀ����
����Ŀ����ⷨ�������Ժ�����ˮ(��Ҫ����Cr2O![]() )ʱ�����������������������������д��ڷ�ӦCr2O
)ʱ�����������������������������д��ڷ�ӦCr2O![]() ��6Fe2����14H��===2Cr3����6Fe3����7H2O�����Cr3����Cr(OH)3��ʽ��ȥ������˵������ȷ����(����)
��6Fe2����14H��===2Cr3����6Fe3����7H2O�����Cr3����Cr(OH)3��ʽ��ȥ������˵������ȷ����(����)
A. ������ӦΪFe��2e��===Fe2��
B. ����������ҺpH����仯
C. ������������Fe(OH)3��������
D. ÿת��12 mol���ӣ���1 mol Cr2O![]() ����ԭ
����ԭ
���𰸡�B
��������FeΪ��������������Ϊ����ʧ���ӣ�ѡ��A��ȷ������������ӦΪ��2H++2e-=H2��������������Ũ�ȵļ�С����Һ��pHһ������������ѡ��B���������Һ�е�Cr3+��Fe3+�����������������ʽ����Һ�г�����ȥ��ѡ��C��ȷ��ÿת��12 mol���ӣ�������6molFe2+�����ݷ���ʽ�������1 mol Cr2O![]() ����ԭ��ѡ��D��ȷ��
����ԭ��ѡ��D��ȷ��

����Ŀ��ij����С�����������ʵ��ⶨCu��Ag�Ͻ���Ũ����ķ�Ӧ����ʵ��װ����ͼ��
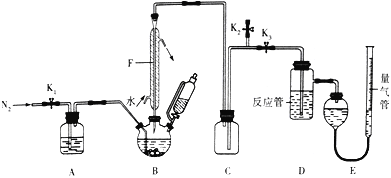
��ش��������⣺
��1��F������������_______________��������____________________________��
��2��ʵ������NH4Cl����ͱ���NaNO2��Һ�ڼ����������Ʊ�N2���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��װ��A��ʢ�ŵ������Ը��������Һ��������___________________________________________��
��3������ʵ�顣���װ�������Ժ�,��װ����������Ӧ���Լ�,Ȼ��__________����������������ٽ�Bװ�÷�Һ©���е�Ũ���Ỻ���μӵ�������ƿ�С�
��4���ⶨ��������ʵ�������Ӧ����������װ��D������500mL��Һ��ÿ��ȡ��25.00mL��Һ������2��ָʾ������0.05 mol��L-1��NaOH��Һ�ζ������εζ������������±���
�ζ�ǰ���/mL | �ζ������/mL | |
��һ�� | 0.33 | 20.32 |
�ʶ��� | 1.25 | 23.26 |
��=.�� | 1.47 | 21.48 |
װ��D�����ɵ�����Ϊ________mol����Cu-Ag�Ͻ���Ũ���ᷴӦ���������ɵ�NO2�ڱ�״���µ����Ϊ__________mL��
��5���ⶨNO��������ڲⶨNO�����ʱ����E����������ˮ��Һ��ȸ���ܵ�Һ��ߣ�ֱ�Ӷ�����ʹ�ⶨ���������_________���ƫ��ƫС��������ʱӦ������Ͳ��λ��_______������ơ������ơ������Ա�֤����Ͳ�е�Һ���������е�Һ���ƽ��