��Ŀ����
����Ŀ������ĿǰӦ�����Ľ��������ĵ��ʼ��仯������;�dz��㷺��
��1��K4[Fe(CN)6]������ʳ�εĿ��������̬��ԭ�ӵĵ����Ų�ʽΪ_______________��
��2��Na2[Fe(CN)5(NO)] ���������Ƹ�Ѫѹ��֢��
��Na��N��O�ĵ�һ��������С�����˳��Ϊ_______________��
��CN-��̼ԭ�ӵ��ӻ�������_______________��
��3������������FeCl3�������·ֽ�����ˮ��������
��1molH2O2����������ĿΪ_______________��
��H2O�ķе��H2S�ߵ�ԭ����_______________��
��4������ý����Ҫ�Ĵ�����CO��������ý���õ�����ʧȥ�����ԣ�Fe+5CO=Fe(CO)5����ȥCO�Ļ�ѧ����ʽΪ[Cu(NH3)2]OOCCH3+CO+NH3=[Cu(NH3)3(CO)]OOCCH3��
����CO��Ϊ�ȵ�����ķ���Ϊ_____________�������[Cu(NH3)2]OOCCH3�в����ڵĵ���������_______������ĸ����
a.���Ӽ� b.������ c.��λ�� d.���Թ��ۼ�
��Fe(CO)5�ڿ�����ȼ�յĻ�ѧ����ʽΪ4Fe(CO)5+13O2 ![]() 2Fe2O3+20CO2��Fe2O3�ľ���������___________��
2Fe2O3+20CO2��Fe2O3�ľ���������___________��
�����ľ�����ͼ��ʾ�����þ�����ܶ���ag��cm-3�������������Feԭ�Ӽ�ľ���Ϊ_____cm����NAΪ����٤��������ֵ����

���𰸡� 1s22s32p63s23p64s1��[Ar]4s1 Na<O<N sp 3NA(��1.806��1024) ˮ���Ӽ�������������ȷ��Ӽ�������ǿ N2 b ���Ӿ��� ![]() ��
��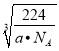
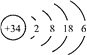
����������1��K��19��Ԫ�أ���̬��ԭ�ӵĵ����Ų�ʽΪ1s22s32p63s23p64s1��[Ar]4s1����2����NaΪ�����������ֻ��һ�����ӣ���ʧȥһ�������γ��ȶ��ṹ�������е�һ��������С��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ�����������������VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ� N��OԪ�ش���ͬһ������ԭ������������N���ڵ�VA�壬���Ե�һ������N��O���ʴ�Ϊ��N��O��Na����CN-Ϊֱ���ͣ�̼ԭ�ӵ��ӻ���ʽΪsp����3����ÿ��H2O2�к���3����������1molH2O2����������ĿΪ3NA��1.806��1024����H2O�ķе��H2S�ߵ�ԭ���ǣ�ˮ���Ӽ�������������ȷ��Ӽ�������ǿ����4���ٵȵ�������ָ������ͬ�۵�����Ŀ��ԭ����Ŀ�ķ��ӻ����ӣ���CO��Ϊ�ȵ�����ķ���ΪN2�������[Cu(NH3)2]OOCCH3�д��ڵĵ������������Ӽ�����λ�������Թ��ۼ��������ڽ���������ѡb����Fe2O3���������Ӻ������ӹ��ɵ����Ӿ��壻�����ľ�����ͼ��ʾ�����þ�����ܶ���ag��cm-3���辧���ı߳�Ϊxcm,�����������Feԭ�Ӽ�ľ���Ϊ![]() xcm�����ݾ�̯����֪һ���������е���ԭ����Ϊ8��
xcm�����ݾ�̯����֪һ���������е���ԭ����Ϊ8��![]() +6��
+6��![]() =4�����������V=x3cm3=
=4�����������V=x3cm3=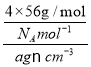 �����x=
�����x= �������������Feԭ�Ӽ�ľ���Ϊ
�������������Feԭ�Ӽ�ľ���Ϊ![]() xcm=
xcm=![]() ��
��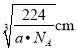 ��
��