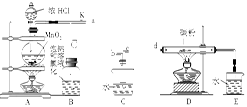��Ŀ����
��ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸����)��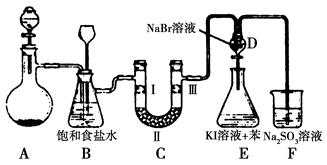
(1)�Ʊ�����ѡ�õ�ҩƷΪƯ�۾������Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ________��
(2)װ��B�б���ʳ��ˮ��������________��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����__________________��
(3)װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η������ʵ������________(����)��
| ��� | a | b | c | d |
| �� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| �� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| �� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
(4)���װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ���Ӧһ��ʱ���������װ��D��������Һ����װ��E�У����۲쵽��������________������________(��ܡ����ܡ�)˵����ķǽ�����ǿ�ڵ⣬ԭ����__________________________��
(5)���������װ��F�пɸ���������NaHSO3��Һ�������ȣ���д����Ӧ�����ӷ�Ӧ����ʽ��__________________�����жϸ���NaHSO3��Һ�Ƿ���У�________(��ǡ���)��
(1)Ca(ClO2)��4HCl(Ũ)=CaCl2��2Cl2����2H2O
(2)��ȥCl2�е�HCl��B�г���©����Һ���������γ�ˮ��
(3)d
(4)E����Һ��Ϊ���㣬�ϲ�(����)Ϊ�Ϻ�ɫ�����ܡ�������Cl2Ҳ�ɽ�I������ΪI2
(5)HSO3����Cl2��H2O=SO42����2Cl����3H��(��4HSO3����Cl2=SO42����2Cl����3SO2��2H2O)����
����

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�S2C12��һ�ֽ��ɫ�ӷ���Һ�壬������������ij��ѧ��ȤС�������ʵ���Ʊ�������S2C12����������֪S2Cl2��ˮ�������绯��Ӧ��һ������Ԫ�ػ��ϼ����ߣ���һ���ֻ��ϼ۽��ͣ����������������ʺ��������Cl2��Ӧ��������S2C12����Ӧ�Ļ�ѧ����ʽΪ��2S+Cl2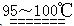 S2Cl2��
S2Cl2��
��Ӧ�漰�ļ������ʵ��۷е����£�
| ���� | S | S2Cl2 |
| �е�/�� | 445 | 138 |
| �۵�/�� | 113 | -76 |
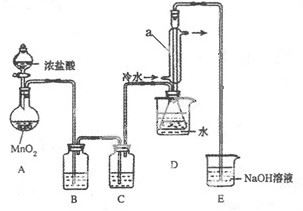
�ش��������⣺
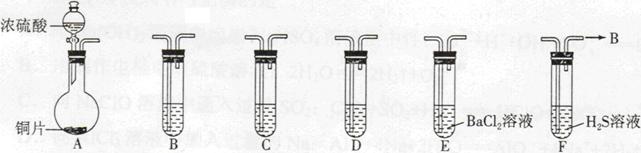
��1�����Ӻ�ʵ��װ�ú�ĵ�һ��ʵ�������______��
��2��ʵ������Ҫ���ȵ������� ����д��ĸ��
��3��װ��B��C�е��Լ��ֱ��� ��
��4��װ��D������a�������� ��
��5����Ӧ���������ƿ�ڻ�����з������Ʒ�ķ�����____________��
��6����ʵ�������ȱ��Cװ�ã����ֲ�Ʒ���Dz��壬���û�ѧ����ʽ��ʾ��ԭ��____________��
��7��ʵ����ϣ�С���е�һλͬѧ��ʣ��Ũ���ᵹ��E�ձ��У������л���ɫ�ݼ�����������������ӷ���ʽ��ʾ�����������ԭ��____________��
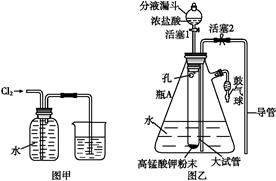
ij������ȤС��Ϊ̽��ͭ��Ũ���ᷴӦ���������ͼ��ʾװ�ý���ʵ�顣��֪����SO2�����ڱ���������������Һ����SO2�������Ը��������Һ����������ԭ��Ӧʹ֮��ɫ����ѧ����ʽΪ5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4����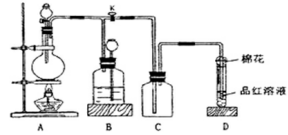
�ش��������⣨ע��EΪֹˮ�У�FΪ��������
��1�����Aװ�õ������Եķ��� ��
��2��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ ��
��3��װ��D���Թܿڷ��õ���Ӧպ��NaOH��Һ��
�������� ��
��4��װ��B����������������á���D�������Ե�����ر�����F����ȥ�ƾ��ƣ��������ȵ����ã�A�����������������ʱB�е������� ��B��Ӧ���õ�Һ���ǣ�����ĸ�� ��
| A��ˮ | B������NaHSO3��Һ | C������KMnO4��Һ | D��NaOH��Һ |
ʵ���Ҳⶨ�����Һ��I���ĺ����Լ���Ļ��չ������£�
��.�����Һ��I�������IJⶨ
����Һ����ȡ25.00 mL��Һ��250 mL��ƿ�У��ֱ����5 mL 2 mol��L��1 H2SO4��10 mL 20% NH4Fe(SO4)2��12H2O��Һ��ҡ�ȡ�С���������������ȫ������ȡ����ƿ��ȴ����10 mL 2 mol��L��1H2SO4�����뼸�ζ�����������(����ָʾ��)����0.0250 mol��L��1��K2Cr2O7��Һ���еζ����յ㡣�ظ�3�Σ����ݼ�¼���±���
(��֪��Ӧ����2Fe3����2I��=2Fe2����I2����6Fe2����Cr2O72-��14H��=6Fe3����2Cr3����7H2O)
| ���� | 1 | 2 | 3 |
| ����(mL) | 19.60 | 19.65 | 19.55 |
��.��Ļ���
ȡ250 mL�����Һ���ձ��м��밴�������������Na2S2O3��Һ������CuSO4������Һ�ڲ��Ͻ����µμӵ���Һ�У�������70��������ȫ��Ӧ(��֪��2I����2Cu2����2S2O32-=2CuI����S4O62-)�����ˣ��õ��ij�����ͼ1���в��������װ�õ������Ժӷ�Һ©������μ���Ũ����(ע���Һ���ٶ�)��ȫ��Ӧ��ͨ����ѹ���ˣ��õ��ֵ�����Ʒ�ͳ���Һ��Ȼ��ͼ2���дֵ���ᴿ��
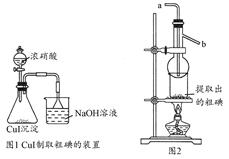
�ش��������⣺
(1)����Һ����ȡ��Һʱ������Һ����ֱ��������б����ƿ�У����ŵIJ�����______________________________________�������ȡ����Һ�ܡ�
(2)��ʢ�з�Һ����ƿ���ȼ���5 mL 2 mol��L��1 H2SO4��Ŀ����__________________________��
(3)���ݵζ��й����ݣ��÷�Һ��I��������________g��L��1��
�ڵζ������У����в���(����������ȷ)����ɲⶨ���ƫ�͵���_____��
A���յ����ʱ���Ӷ������ζ�ǰƽ�Ӷ���
B����ƿˮϴ��δ����
C���ζ���δ�ñ�K2Cr2O7��Һ��ϴ
D��ʢ��K2Cr2O7��Һ�ĵζ��ܣ��ζ�ǰ�����ݣ��ζ���������
(4)��ͼ1��ƿ�з�����Ӧ�Ļ�ѧ����ʽ��____________________��
(5)��ͼ2װ�ý��дֵ��ᴿ�����õķ��뷽����________��a��bΪ����ˮ�����ڣ�����________(ѡ��a��b)��ˮ��ͷ��ˮ�����յõ��ϸߴ��ȵĵ��ʵ⡣
ij����С��������CuO��NH3��Ӧ,�о�NH3��ij�����ʲ��ⶨ�����,���������ʵ��װ��(�г�װ��δ����)����ʵ�顣��ش���������: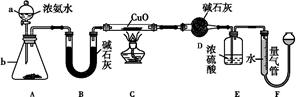
(1)����a������Ϊ��������;����b�п�ѡ����Լ�Ϊ����������
(2)ʵ������,����װ��A,������ȡ����ɫ�������������� (����ĸ)��
| A��Cl2 | B��O2 | C��CO2 | D��NO2 |
(4)Eװ����Ũ������������� ��
(5)��ȡ�������ǰ,Ӧ��װ��F���еIJ���: ����
(6)ʵ�����,����ø����D����m g,װ��F�����������Ϊn L(������ɱ�״��),�����е������ԭ�Ӹ�����Ϊ��������(�ú�m��n��ĸ�Ĵ���ʽ��ʾ)��
��ͼ5��װ�ö�����ѧ��ѧ�г�����ʵ��װ�ã�ijѧϰС���ͬѧ������Щװ�ý��г������ʵ���ȡ��̽�������ʣ�ͼ��a��b��c��ʾֹˮ�У��������������ƻ����ۣ��Իش��������⣺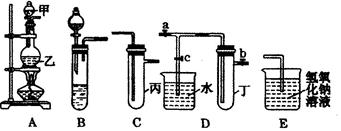
��1����A��C��E��������Ũ����� ����д���ƣ�Ϊԭ����ȡCl2�������ҵ������� ��
��2�����ã�1����װ�ú�ҩƷ���ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ�飬ʵ����������������£�
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ��������ˮ����Ʒ����Һ | Ʒ����Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��������ˮ�м���̼�����Ʒ�ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ���ٲ���һ������ǿ��̼������� |
ʵ����ý����Ƿ������ �����������������������ѡ��������������˵�����ɣ���ѡ������������������д���ɣ��� ��
��3�����ã�1����װ�û������һ����ʵ��Ƚ�Cl����S2���Ļ�ԭ��ǿ������C��Ԥ�ڳ��ֵ������� ��
��4����B��D��Eװ�������ӣ���ֹˮ��a��ֹˮ��b���ر�ֹˮ��c������B��ʢװŨ�����ͭƬ����ͭƬ�����п����ϰ��ϣ������Ƶ�NO2��һ��ʱ�������Dװ��̽��NO2��ˮ�ķ�Ӧ�����������Ϊ���� ���� ��ʹ�ձ��е�ˮ�����Թܶ����۲�����