��Ŀ����
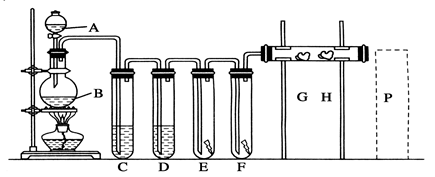
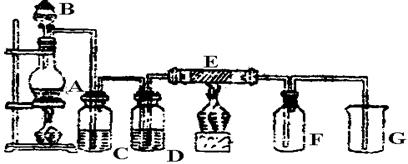
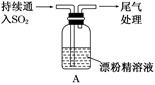
��ˮ��ʵ���ҳ�����ʱ���Ƶ�һ���Լ�,ͼ����ʾ����ʵ����������ˮʱ��һ�ֳ���װ��,ͼ����ij��ѧʵ��С��������Ƶ�һ��������ˮ��װ��(ͼ�еĹ�������һ�־������嵥�ġ���������ƿ�й������������)��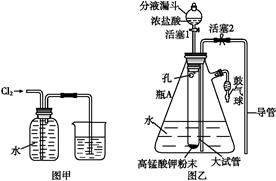
(1)��ˮ����ʱ���Ƶ�ԭ����: ��(�û�ѧ����ʽ��ʾ)��
(2)ͼ����ʾװ����,�ձ��ڵ�Һ��������������
(3)ͼ����ʾװ����,ƿA �ڵĴ��Թ��϶˹ܱ�����һ��С��,ƿA�����ƽ���������,������ͨ������
��������ʵ����(�����ʵ�����������)��
(4)��ͼ����ʾװ��������ˮʱ,������������:
�ٹرջ���2,��Һ©���ϿڵIJ�����,�ٴ���1,������Ũ����ע����Թ��ڡ�
�ڹرջ���1,���Ϸ�Һ©���Ͽڲ�������Ũ����ʹ��Թ��ڵĸ�����ط�ĩ��Ӧ����������
������ҡ��ƿA,ʹ������������ˮ�С�
��ͼ��װ�����ڽ϶�ʱ���ڵõ�������ˮ��������ҡ�������������������ˮ�ĽӴ�������,��һ����Ҫԭ���� ��
��Һ©���е�����Ӧ�����μ����Թ��ڡ���һ�μ���̫�������,��������ĺ���� ��
(5)������ˮ��ɺ�,���ز�жװ�ü��ɴ�ƿA��ȡ��������ˮ,������ ��
(1)2HClO 2HCl+O2��
2HCl+O2��
(2)NaOH��Һ��(3)����ƿ����ѹ����
(4)������ƿ�ڲ����ϴ�ѹǿ,�������������ܽ�ȡ�ƿ�ڲ������������,ƿ��ѹǿ�����ʹƿA����Ƥ������
(5)����2,�رջ���1,�ù�������ƿA�й������
����

 ��ս�п�����ϵ�д�
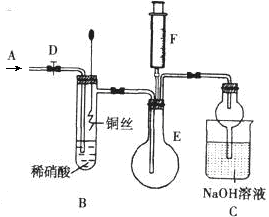
��ս�п�����ϵ�д���ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸����)��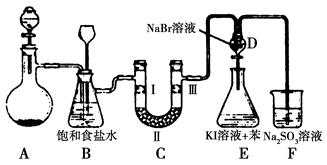
(1)�Ʊ�����ѡ�õ�ҩƷΪƯ�۾������Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ________��
(2)װ��B�б���ʳ��ˮ��������________��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����__________________��
(3)װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η������ʵ������________(����)��
| ��� | a | b | c | d |
| �� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| �� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| �� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
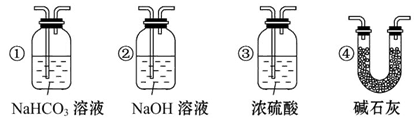
(4)���װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ���Ӧһ��ʱ���������װ��D��������Һ����װ��E�У����۲쵽��������________������________(��ܡ����ܡ�)˵����ķǽ�����ǿ�ڵ⣬ԭ����__________________________��
(5)���������װ��F�пɸ���������NaHSO3��Һ�������ȣ���д����Ӧ�����ӷ�Ӧ����ʽ��__________________�����жϸ���NaHSO3��Һ�Ƿ���У�________(��ǡ���)��
ijѧ����SO2��Ư�۾��ķ�Ӧ����ʵ��̽����
| ���� | ���� |
| ȡ4 gƯ�۾����壬����100 mLˮ | ���ֹ����ܽ⣬��Һ������ɫ |
| ���ˣ���Ư�۾���Һ��pH | pH��ֽ�ȱ���(ԼΪ12)������ɫ |
 | i.Һ���Ϸ����ְ����� ii.�Ժ��ֻ��ǣ���Һ��Ϊ����ɫ��iii.�Ժ���������ɫ����������ɫ��ȥ |
(1)Cl2��Ca(OH)2��ȡƯ�۾��Ļ�ѧ����ʽ�� ________________________________��
(2)pH��ֽ��ɫ�ı仯˵��Ư�۾���Һ���е�������__________________________��
(3)��ˮ�г���ͨ��SO2��δ�۲쵽�������Ʋ�����i�İ�����HClСҺ���γɡ���������ʵ�飺
a����ʪ��ĵ⻯�ص�����ֽ����������ޱ仯��
b�����ữ��AgNO3��Һ���������������ɫ������
�� ʵ��a��Ŀ����______________________________________________________��
����ʵ��a��b�����жϰ����к���HCl��������________________________________��
(4)����ii����Һ��Ϊ����ɫ�Ŀ���ԭ������Һ���Ե���ǿ��Ư�۾�����Ч�ɷֺ�Cl��������Ӧ��ͨ����һ��ʵ��ȷ�������ֿ����ԣ���ʵ�鷽����_____________________��
(5)��Aƿ�л������ˡ�ϴ�ӣ��õ�����X
�������X�м���ϡHCl�������Ա仯��ȡ�ϲ���Һ������BaCl2��Һ��������ɫ�����������X�к��е�������________��
�������ӷ���ʽ��������iii�л���ɫ��ȥ��ԭ��_____________________________________________________________________��
��������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶá���֪��Na2S2O3��������Һ�в����ȶ����ڡ�
��1��ij�о�С����Ƶ��Ʊ�Na2S2O3��5H2O��װ�úͲ��ֲ����������¡�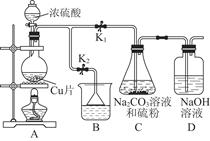
��.��K1�ر�K2����Բ����ƿ�м�������Ũ���ᣬ���ȡ�
��.C�л��Һ��������������Ӧһ��ʱ�����۵������٣���C����Һ��pH �ӽ�7ʱ����K2���ر�K1��ֹͣC�еķ�Ӧ��ֹͣ���ȡ�
��.����C�еĻ��Һ��
��.����Һ���� �� �����ˡ�ϴ�ӡ���ɣ��õ���ƷNa2S2O3��5H2O��
�٢��У�����C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ����ԭ���� �������ӷ���ʽ��ʾ����
�ڢ��У����������� �� ��
��װ��B��ʢ�ŵ��Լ��ǣ��ѧʽ�� ��Һ��
����һС����ʵ���з��֣�����������������������º����������ԣ����ֲ��������������⣬�����Ʋ���ܵ�ԭ�� ��
��2������Na2S2O3��Һ�ⶨ��ˮ��Ba2����Ũ�ȣ��������£�ȡ��ˮ25.00 mL�������ʵ�����ȼ������� K2Cr2O7��Һ����BaCrO4���������ˡ�ϴ�Ӻ�������ϡ�����ܽ⣬��ʱCrO42-ȫ��ת��ΪCr2O72-���ټӹ�KI��Һ����ַ�Ӧ��û����ҺV mL������ƽ���ֳ�4�ȷݣ����������Һ��ָʾ������0.001 0 mol��L��1��Na2S2O3��Һ���еζ�����Ӧ��ȫʱ��������ݼ�¼���±���ʾ��
| ��� | 1 | 2 | 3 | 4 |
| ����Na2S2O3�� | | | | |
| ��Һ�����/mL | 18.02 | 17.98 | 18.00 | 20.03 |
���ַ�Ӧ���ӷ���ʽΪ��
��Cr2O72-��6I����14H��=2Cr3����3I2��7H2O��
��I2��2S2O32-=2I����S4O62-��
���жϴﵽ�ζ��յ�������� ����ˮ��Ba2�������ʵ���Ũ�� _��