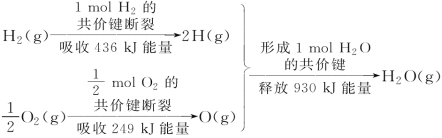
��Ŀ����
����Ŀ���������������������dz����Ĵ�����Ⱦ�
��1�������ǽ�ˮ������(pHС��5.6)��ͳ�ƣ�����ط���������2.1(ʳ��pH��3)�������������������Ǵ����е����������͵��������������ǵ���Ҫ��Դ��ú��ʯ�͵�ȼ�ա�
�������ŷŵ�β�������᳧�ͻ��ʳ��ķ����ж����е��������ȫ����ÿ���ŷ���ԼΪ5��107 kg��NO2����ˮ����_____________��д���ƣ���NO��
��Ϊ�˼���������γɣ��������SO2���ŷ�������ȼ���е���Ԫ�ؽ��й̻��������Է����еĵ�����������__________���գ�д��NO2��֮��Ӧ�����ӷ���ʽ____________________________��
��2��ij��ѧ��ȤС��ѡ����ͼʵ��װ�ã��ⶨ��ҵԭ����(��SO2��N2��O2)��SO2�ĺ�����
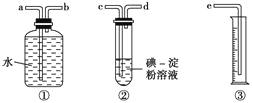
����ԭ��������������ʱ������װ�����ӵ�˳���ǣ�ԭ������ �� �� �� �� (����ĸ)��_________
��װ�����з�����Ӧ�����ӷ���ʽΪ��___________________________________________����װ�����г���_____________________________����ʱ������ֹͣͨ����
������Ϊ�����Լ��У��������������Թ��еĵ�ĵ�����Һ����__________��
A������KMnO4��Һ B��NaOH��Һ C����ˮ D����ˮ
���𰸡� ���� ����Һ��NaOH��Һ�� 2NO2+2OH-=NO2-+NO3-+H2O cdbae SO2+I2+2H2O=4H++2I-+SO42- ��Һ��ɫ�պ���ȥ AC
����������1����NO2����ˮ���������NO����ȷ����������
�������������ܹ�������������Һ��Ӧ�����Σ��ﵽ����Ŀ�ģ�����������NO2��Ӧ���������ƺ��������������������ӷ���ʽ��2NO2+2OH-=NO2-+NO3-+H2O����ȷ��������Һ��NaOH��Һ���� 2NO2+2OH-=NO2-+NO3-+H2O��
��2�������ڵ�����������������ˮ������ͨ����ˮ������������������ȷ��˳��Ϊcdbae����ȷ����cdbae��
���ⵥ�ʾ������������Ѷ�����������Ϊ������������ԭΪ�����ӣ����ڵ�����������ɫ�����ⵥ�ʷ�Ӧ��ȫ������ɫ��Һǡ�ñ�Ϊ��ɫ������ֹͣͨ������Ӧ�����ӷ���ʽΪ��SO2+I2+2H2O=4H++2I-+SO42- ����ȷ�𰸣�cdbae�� SO2+I2+2H2O=4H++2I-+SO42-����Һ��ɫ�պ���ȥ��
������KMnO4��Һ����ˮ�����������ԣ�����Һ������ɫ�������ܹ����棬NaOH��Һ�Ͱ�ˮ�������������Ӧ������û����A ��C ��ȷ����ȷѡ��A�� C��

����Ŀ��A��B��C��D��E��F��ԭ��������������Ķ���������Ԫ�أ�A��E��Ԫ�����ڱ��е����λ����ͼ��A����Ԫ�����γ�������ɫ���壬C�ǵؿ��к�������Ԫ�أ�D�ǵؿ��к������Ľ���Ԫ�أ�
A | ||
E |
��1��C��Ԫ�����ڱ��е�λ��Ϊ �� �����ӵĽṹʾ��ͼΪ ��
��2��AE2�ķ���ʽΪ ��
��3��C��E��F�ĵ��ʷе���͵������ѧʽ����
��4��C��D��E��F�����Ӱ뾶�ɴ�С��˳�����������ӷ��ţ���
��5��ʵ������ȡF2��������ӷ���ʽΪ
��6�������ӹ�ҵ�У�B�������̬�⻯���ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ ��