��Ŀ����
����Ŀ����Ǧ�����Ҫ�ɷ�ΪPbS����Ǧ�ɴ�86.6%���Է�Ǧ��Ϊԭ���Ʊ�Ǧ���صĵ缫����PbO2�Ĺ���������ͼ��ʾ��

��1����Ǧ���պ��ų��������Ҫ�ɷ���______���ѧʽ���������ڹ�ҵ�Ʊ�________��
��2��������н�̿��������________��
��3������Ǧ����������Ҫ��п������ͭ�����ȡ���⾫��ʱ��________��������������ӦʽΪ________�����������Ҫ�ɷ���________��
��4����������Ʊ�PbO2�����ӷ���ʽ________��
��5����֪�������£�Ksp(PbS)=8��10-28��Ka1(H2S)=1.3��10-7��Ka2(H2S)=7.1��10-15�������£���Pb(NO3)2��Һ��ͨ��H2S���壬������Ӧ�����ӷ���ʽ��________��������ݷ������÷�Ӧ�ܷ���еû�����ȫ��________��д�������������̣���
���𰸡� N2�� SO2 ���ᣨ ������ �� ���� ��ԭ����Ǧ ��Ǧ Pb2++2e��=Pb Cu�� Ag PbO+ClO��=PbO2+Cl�� Pb2++H2S=PbS��+ 2H+ �÷�Ӧ��ƽ�ⳣ����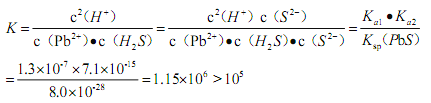 �����Է�Ӧ�ܽ��еû�����ȫ��
�����Է�Ӧ�ܽ��еû�����ȫ��
����������1����Ǧ��������PbO��SO2�ȣ��ų��������Ҫ�ɷ���SO2�Լ�������δ��Ӧ��N2���������N2�����ڹ�ҵ�Ʊ������������������������������2��������к��ж���Ǧ�Ĺ����ڸ����������뽹̿��Ӧ�õ���Ǧ����̿�������ǻ�ԭ����Ǧ����3������Ǧ����������Ҫ��п������ͭ�����ȡ���⾫��ʱ����Ǧ��������������ӦʽΪPb2++2e��=Pb�����������Ҫ�ɷ���Cu��Ag����4���������PbO��������Ʒ�Ӧ�Ʊ�PbO2�����ӷ���ʽΪPbO+ClO��=PbO2+Cl������5����Pb(NO3)2��Һ��ͨ��H2S��������PbS������������Ӧ�����ӷ���ʽ��Pb2++H2S=PbS��+ 2H+��������ݷ������÷�Ӧ��ƽ�ⳣ����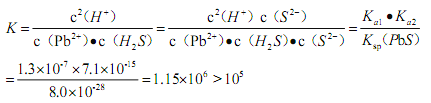 �����Է�Ӧ�ܽ��еû�����ȫ��
�����Է�Ӧ�ܽ��еû�����ȫ��