��Ŀ����
��Ļ������������Ϳ����з�������Ҫ���á�
��1��SO2Cl2����������ҽҩƷ��Ⱦ�ϡ�������Լ��ȡ���֪��SO2Cl2(g) SO2(g)��Cl2(g) ��H����97.3 kJ��mol��1��ij�¶�ʱ�����Ϊ1 L�ĺ����ܱ������г���0. 20mol SO2Cl2���ﵽƽ��ʱ�������к�0.18mol SO2����˹��̷�Ӧ���յ�����Ϊ_____ kJ�����¶�ʱ��Ӧ��ƽ�ⳣ��Ϊ_____�����������û��������������BaCl2��Һ�У��������ɳ���������Ϊ_______��
SO2(g)��Cl2(g) ��H����97.3 kJ��mol��1��ij�¶�ʱ�����Ϊ1 L�ĺ����ܱ������г���0. 20mol SO2Cl2���ﵽƽ��ʱ�������к�0.18mol SO2����˹��̷�Ӧ���յ�����Ϊ_____ kJ�����¶�ʱ��Ӧ��ƽ�ⳣ��Ϊ_____�����������û��������������BaCl2��Һ�У��������ɳ���������Ϊ_______��
��2����ҵ���Ʊ�����Ĺ����д��ڷ�Ӧ��2SO2(g)��O2(g) 2SO3(g) ��H����198kJ��mol��1��400�棬1.01��105Pa�����ݻ�Ϊ2L�ĺ����ܱ������г���һ���� SO2��O2��n(SO3)��n(O2)��ʱ��ı仯������ͼ��ʾ��
2SO3(g) ��H����198kJ��mol��1��400�棬1.01��105Pa�����ݻ�Ϊ2L�ĺ����ܱ������г���һ���� SO2��O2��n(SO3)��n(O2)��ʱ��ı仯������ͼ��ʾ��

��0��20min��Ӧ��ƽ�����ʦ�(O2)��___________��
������������ȷ���� ��
a��A�����(SO2)������(SO2)
b��B�㴦��ƽ��״̬
c��C���D��n(SO2)��ͬ
d�������������䣬500��ʱ��Ӧ��ƽ�⣬n(SO3)��ͼ��D���ֵ��
��3����ҵ����Na2SO3��Һ���������е�SO2��������ͨ��1.0 mol��L��1��Na2SO3��Һ������ҺpHԼΪ6ʱ��Na2SO3��Һ����SO2�����������½���Ӧ�������ռ�����ʱ��Һ��c (SO32��)��Ũ����0.2 mol��L��1������Һ��c(HSO3��)��__mol��L��1��
��1��SO2Cl2����������ҽҩƷ��Ⱦ�ϡ�������Լ��ȡ���֪��SO2Cl2(g)
 SO2(g)��Cl2(g) ��H����97.3 kJ��mol��1��ij�¶�ʱ�����Ϊ1 L�ĺ����ܱ������г���0. 20mol SO2Cl2���ﵽƽ��ʱ�������к�0.18mol SO2����˹��̷�Ӧ���յ�����Ϊ_____ kJ�����¶�ʱ��Ӧ��ƽ�ⳣ��Ϊ_____�����������û��������������BaCl2��Һ�У��������ɳ���������Ϊ_______��
SO2(g)��Cl2(g) ��H����97.3 kJ��mol��1��ij�¶�ʱ�����Ϊ1 L�ĺ����ܱ������г���0. 20mol SO2Cl2���ﵽƽ��ʱ�������к�0.18mol SO2����˹��̷�Ӧ���յ�����Ϊ_____ kJ�����¶�ʱ��Ӧ��ƽ�ⳣ��Ϊ_____�����������û��������������BaCl2��Һ�У��������ɳ���������Ϊ_______����2����ҵ���Ʊ�����Ĺ����д��ڷ�Ӧ��2SO2(g)��O2(g)
 2SO3(g) ��H����198kJ��mol��1��400�棬1.01��105Pa�����ݻ�Ϊ2L�ĺ����ܱ������г���һ���� SO2��O2��n(SO3)��n(O2)��ʱ��ı仯������ͼ��ʾ��
2SO3(g) ��H����198kJ��mol��1��400�棬1.01��105Pa�����ݻ�Ϊ2L�ĺ����ܱ������г���һ���� SO2��O2��n(SO3)��n(O2)��ʱ��ı仯������ͼ��ʾ��
��0��20min��Ӧ��ƽ�����ʦ�(O2)��___________��
������������ȷ���� ��
a��A�����(SO2)������(SO2)
b��B�㴦��ƽ��״̬
c��C���D��n(SO2)��ͬ
d�������������䣬500��ʱ��Ӧ��ƽ�⣬n(SO3)��ͼ��D���ֵ��
��3����ҵ����Na2SO3��Һ���������е�SO2��������ͨ��1.0 mol��L��1��Na2SO3��Һ������ҺpHԼΪ6ʱ��Na2SO3��Һ����SO2�����������½���Ӧ�������ռ�����ʱ��Һ��c (SO32��)��Ũ����0.2 mol��L��1������Һ��c(HSO3��)��__mol��L��1��
��1��17.5 1.62 46.6g
��2��0.02mol/(L��min) ac
��3��1.6mol/L
��2��0.02mol/(L��min) ac
��3��1.6mol/L
��1���ﵽƽ��ʱ�������к�0.18mol SO2��������Ȼ�ѧ����ʽ��֪���˹��̷�Ӧ���յ�����Ϊ97.3 kJ��mol��1��0.18mol��17.5kJ�����ݷ���ʽ��֪
SO2Cl2(g) SO2(g)��Cl2(g)
SO2(g)��Cl2(g)
��ʼŨ�ȣ�mol/L�� 0.20 0 0
ת��Ũ�ȣ�mol/L�� 0.18 0.18 0.18
ƽ��Ũ�ȣ�mol/L�� 0.02 0.18 0.18
���Ը��¶��·�Ӧ��ƽ�ⳣ��K��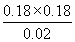 ��1.62��
��1.62��
���û��������������BaCl2��Һ�У�������ӦSO2��Cl2��2H2O��H2SO4��2HCl�����Դ�ʹƽ�����������Ӧ������У�����������ɳ��������ʵ�����0.20mol�������ᱵ������Ϊ0.20mol��232g/mol��46.6g��
��2���ٸ���ͼ���֪20minʱ������������ʵ�����1.6mol�����������������ʵ�����0.8mol��Ũ����0.4mol/L������0��20min��Ӧ��ƽ�����ʦ�(O2)��0.4mol/L��20min��0.02mol(L��min)��
��a������ͼ���֪A�㷴Ӧû�дﵽƽ��״̬��ƽ��������Ӧ������У������(SO2)������(SO2)��a��ȷ��b��B�����ʵ�Ũ����Ȼ�DZ仯�ģ���Ӧû�д���ƽ��״̬��b����ȷ��c��C���D�������ͬ�����µ�ƽ��״̬�����n(SO2)��ͬ��c��ȷ��d������Ӧ�Ƿ��ȷ�Ӧ�������������䣬�����¶�ƽ�����淴Ӧ�����ƶ�������500��ʱ��Ӧ��ƽ�⣬n(SO3)��ͼ��D���ֵС��d����ȷ����ѡac��
��3����Һ��c (SO32��)��Ũ����0.2 mol��L��1��������c (SO32��)��Ũ�ȣ�1.0mol/L��0.2mol/L��0.8mol/L����˸��ݷ���ʽSO32����SO2��H2O��2HSO3����֪��Һc(HSO3��)��0.8mol/L��2��1.6mol/L
SO2Cl2(g)
 SO2(g)��Cl2(g)
SO2(g)��Cl2(g)��ʼŨ�ȣ�mol/L�� 0.20 0 0
ת��Ũ�ȣ�mol/L�� 0.18 0.18 0.18
ƽ��Ũ�ȣ�mol/L�� 0.02 0.18 0.18
���Ը��¶��·�Ӧ��ƽ�ⳣ��K��
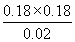 ��1.62��
��1.62�����û��������������BaCl2��Һ�У�������ӦSO2��Cl2��2H2O��H2SO4��2HCl�����Դ�ʹƽ�����������Ӧ������У�����������ɳ��������ʵ�����0.20mol�������ᱵ������Ϊ0.20mol��232g/mol��46.6g��
��2���ٸ���ͼ���֪20minʱ������������ʵ�����1.6mol�����������������ʵ�����0.8mol��Ũ����0.4mol/L������0��20min��Ӧ��ƽ�����ʦ�(O2)��0.4mol/L��20min��0.02mol(L��min)��
��a������ͼ���֪A�㷴Ӧû�дﵽƽ��״̬��ƽ��������Ӧ������У������(SO2)������(SO2)��a��ȷ��b��B�����ʵ�Ũ����Ȼ�DZ仯�ģ���Ӧû�д���ƽ��״̬��b����ȷ��c��C���D�������ͬ�����µ�ƽ��״̬�����n(SO2)��ͬ��c��ȷ��d������Ӧ�Ƿ��ȷ�Ӧ�������������䣬�����¶�ƽ�����淴Ӧ�����ƶ�������500��ʱ��Ӧ��ƽ�⣬n(SO3)��ͼ��D���ֵС��d����ȷ����ѡac��
��3����Һ��c (SO32��)��Ũ����0.2 mol��L��1��������c (SO32��)��Ũ�ȣ�1.0mol/L��0.2mol/L��0.8mol/L����˸��ݷ���ʽSO32����SO2��H2O��2HSO3����֪��Һc(HSO3��)��0.8mol/L��2��1.6mol/L

��ϰ��ϵ�д�
 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
�����Ŀ
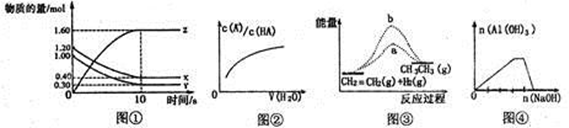
 Z(g)����60s�ﵽƽ�⣬����0.3molZ������˵����ȷ���ǣ� ��
Z(g)����60s�ﵽƽ�⣬����0.3molZ������˵����ȷ���ǣ� ��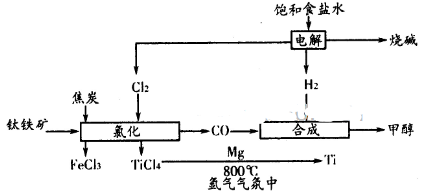
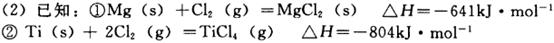
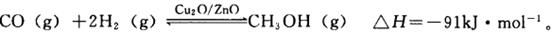
 �Ļ�ѧƽ�ⳣ
�Ļ�ѧƽ�ⳣ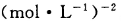 ��
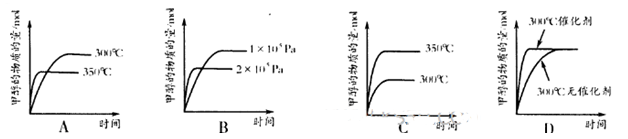
��
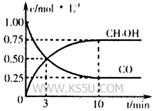
 2NO2��ƽ�⡣��ͼ�Ǹ�һ��ʱ��ⶨ����N2O4��Ũ�ȣ�������ΪN2O4��Ũ�ȣ�������Ϊʱ�䣩
2NO2��ƽ�⡣��ͼ�Ǹ�һ��ʱ��ⶨ����N2O4��Ũ�ȣ�������ΪN2O4��Ũ�ȣ�������Ϊʱ�䣩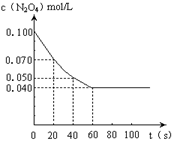
 p C�У�m��n��pΪ�����ʵļ��������ֲ��Cÿ��������a mol/L��Bÿ���Ӽ���1��5a mol/L��Aÿ���Ӽ���0��5a mol/L����m��n��pΪ
p C�У�m��n��pΪ�����ʵļ��������ֲ��Cÿ��������a mol/L��Bÿ���Ӽ���1��5a mol/L��Aÿ���Ӽ���0��5a mol/L����m��n��pΪ CH3OH(g)�������ͼʾ�ش��������⣺
CH3OH(g)�������ͼʾ�ش��������⣺