��Ŀ����
һ���¶��£��� 3 mol SO2�� 1 mol O2 ����һ�����ܱ������У��ڴ��������½������з�Ӧ: 2SO2��g�� + O2��g�� 2SO3��g������H= ��197 kJ/ mol�����ﵽƽ��״̬ʱ������˵������ȷ���ǣ� ��
2SO3��g������H= ��197 kJ/ mol�����ﵽƽ��״̬ʱ������˵������ȷ���ǣ� ��
 2SO3��g������H= ��197 kJ/ mol�����ﵽƽ��״̬ʱ������˵������ȷ���ǣ� ��
2SO3��g������H= ��197 kJ/ mol�����ﵽƽ��״̬ʱ������˵������ȷ���ǣ� ��| A������ SO3Ϊ2 mol | B��SO2�� SO3 ���ʵ���֮��һ��Ϊ 3 mol |
| C���ų� 197 kJ ������ | D��SO2�����ʵ�����SO3 ���ʵ���һ����� |
B
��Ϊ�ǿ��淴Ӧ�����Բ���������2molSO2��A��D����ȷ��������ԭ���غ��֪��B����ȷ�ģ���D��һ����ȷ����ѡB��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����:
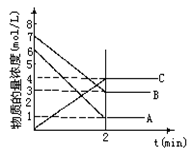
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����: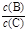 ��_________��
��_________��
 4C
4C H2(g) + I2(g)������ƽ����ϵѹǿ��ʹ��ɫ����
H2(g) + I2(g)������ƽ����ϵѹǿ��ʹ��ɫ����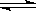 2NH3(g) ��ƽ��ı�־��
2NH3(g) ��ƽ��ı�־�� 2HI(g)������һ������ƽ��״̬����
2HI(g)������һ������ƽ��״̬���� ���ﵽƽ��ʱ��C�����ʵ����ٷֺ���Ϊw����ά������������¶Ȳ��䣬���������ַ����ı���ʼ���ʣ��ﵽƽ���C�����ʵ����ٷֺ�������w����
���ﵽƽ��ʱ��C�����ʵ����ٷֺ���Ϊw����ά������������¶Ȳ��䣬���������ַ����ı���ʼ���ʣ��ﵽƽ���C�����ʵ����ٷֺ�������w����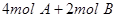
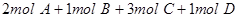
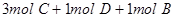
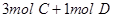
 2HI��g�����������һ����˵����Ӧ�Ѵﵽƽ��״̬���ǣ� ��
2HI��g�����������һ����˵����Ӧ�Ѵﵽƽ��״̬���ǣ� ��