��Ŀ����
��8�֣���ѧѧ���е�ƽ��������Ҫ���ݰ�������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ������֡��Ҿ�������������ԭ������ش��������⡣
(1)һ���¶��£���һ���̶��ݻ����ܱ������У����淴Ӧ
A(g)+2B(g)  4C(g)
4C(g)  H ��0 �ﵽƽ��ʱ��c(A)="2mol/L," c(B)="7mol/L," c(C)=4mol/L����ȷ��B����ʼŨ��c(B)��ȡֵ��Χ�� �����ı��������´ﵽƽ�����ϵ��C����������������д�ʩ���е���
H ��0 �ﵽƽ��ʱ��c(A)="2mol/L," c(B)="7mol/L," c(C)=4mol/L����ȷ��B����ʼŨ��c(B)��ȡֵ��Χ�� �����ı��������´ﵽƽ�����ϵ��C����������������д�ʩ���е���
������C�����ʵ��� �ڼ� ѹ ������ ��ʹ�ô���
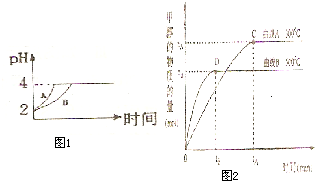
(2)�����£�ȡpH=2������ʹ�����Һ��100mL�������зֱ����������Zn������Ӧ����������Һ��pH�仯��ͼ��ʾ����ͼ�б�ʾ������Һ��pH�仯���ߵ��� ���A����B�������������м����Zn����Ϊm1��������Һ�м����Zn����Ϊm2����m1 m2��ѡ���������=������������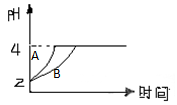
��1��3mol/L��c(B)��9mol/L �� ��2��B����
����

��ϰ��ϵ�д�
�����Ŀ
 ��ѧѧ���е�ƽ��������Ҫ��������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ�����֣��Ҿ�������������ԭ������ش��������⣺
��ѧѧ���е�ƽ��������Ҫ��������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ�����֣��Ҿ�������������ԭ������ش��������⣺ ��ѧѧ���е�ƽ��������Ҫ���ݰ�������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ������֣��Ҿ�������������ԭ������ش��������⣮
��ѧѧ���е�ƽ��������Ҫ���ݰ�������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ������֣��Ҿ�������������ԭ������ش��������⣮ ��ѧѧ���е�ƽ��������Ҫ��������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ�����֣��Ҿ�������������ԭ������ش��������⣺
��ѧѧ���е�ƽ��������Ҫ��������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ�����֣��Ҿ�������������ԭ������ش��������⣺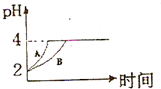 ��ѧѧ���е�ƽ��������Ҫ��������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ�����֣��Ҿ�������������ԭ������ش��������⣺�����£�ȡ pH=2������ʹ�����Һ��100mL�������зֱ����������Zn������Ӧ����������Һ��pH�仯��ͼ��ʾ����ͼ�б�ʾ������Һ��pH�仯���ߵ���
��ѧѧ���е�ƽ��������Ҫ��������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ�����֣��Ҿ�������������ԭ������ش��������⣺�����£�ȡ pH=2������ʹ�����Һ��100mL�������зֱ����������Zn������Ӧ����������Һ��pH�仯��ͼ��ʾ����ͼ�б�ʾ������Һ��pH�仯���ߵ���