��Ŀ����
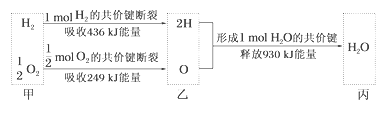
����Ŀ�����Ĺ̶�һֱ�ǿ�ѧ���о�����Ҫ���⣬�ϳɰ������˹��̵��Ƚϳ���ļ�������ԭ��ΪN2 (g)+3H2 (g)![]() 2NH3(g)
2NH3(g)
(1)��֪ÿ�ƻ�1mol�йػ�ѧ����Ҫ���������±���
H-H | N-H | N-N | N��N |
435.9kJ | 390.8kJ | 192.8kJ | 945.8kJ |
(1)��Ӧ���������_________(����>���� ��<��)�������������
(2)��һ���¶��¡���2L�ܱ������м���2 molN2��6 mol H2�����ʵ��Ĵ��������£�������Ӧ N2 (g)+3H2 (g)![]() 2NH3(g)��10min��ﵽƽ�⣬��ʱʣ��4.5mol H2��
2NH3(g)��10min��ﵽƽ�⣬��ʱʣ��4.5mol H2��
������������˵���˷�Ӧ�ﵽƽ��״̬����____��
a����������ѹǿ���� b��v(H2)����v(H2)���� c��N2��H2��Ũ�����
d�� 2 mol NH3���ɵ�ͬʱ��3 moH��H������ e��NH3��Ũ�Ȳ��ٸı�
��0��10 min�ڵ�ƽ����Ӧ����v(H2) ��____mol/(Lmin)��10��ĩNH3��Ũ����___mol/L��N2 �ĵ����ʵ���___mol
��ij�¶�ʱ����һ��2L���ܱ�������X��Y��Z�����������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ���ݴ˻ش�
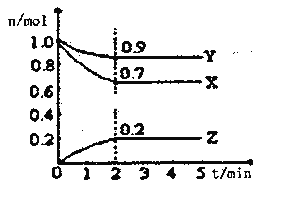
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ___________
(2)�ӿ�ʼ��2min��Z��ƽ����Ӧ����Ϊ____________��
(3)�ı��������������Լӿ컯ѧ��Ӧ���ʵ���_________
A�������¶� B����С����X�����ʵ��� C����Сѹǿ D����������Z�����ʵ���. E������ij�ִ��� F.��С�ݻ� G��ʹ��Ч�ʸ��ߵĴ���
���𰸡��� abe 0.075 0.5 1.5 3X+Y![]() 2Z 0.05 mol/(L��min) ADEFG
2Z 0.05 mol/(L��min) ADEFG
��������
��1��������H=��Ӧ����ܺ�-��������ܺ����㣻
��2���ٴ�ƽ��״̬������ȥ������ƽ���־�����淴Ӧ������ͬ������ֺ������ֲ��䡢Ũ�ȵȲ��䣻
����2L�ܱ������м���2molN2��6mol H2�����ʵ��Ĵ��������£�������Ӧ N2 ��g��+3H2 ��g��![]() 2NH3��g����10min��ﵽƽ�⣬��ʱʣ��4.5mol H2����
2NH3��g����10min��ﵽƽ�⣬��ʱʣ��4.5mol H2����
N2 ��g��+3H2 ��g��![]() 2NH3��g��
2NH3��g��
��ʼ����mol��L��1��1 3 0
ת������mol��L��1��0.25 0.75 0.5
ƽ�⣺��mol��L��1��0.75 2.25 0.5
�Դ˼�����⣮
��1���������ʵ����ı仯�жϷ�Ӧ���������������ʵ����ı仯֮�ȵ��ڻ�ѧ������֮����д����ʽ��
��2������v=��c/��t���㷴Ӧ���ʣ�
��3��Ӱ�컯ѧ��Ӧ���ʵ�������Ũ�ȡ��¶ȡ�ѹǿ�������Լ������������ܼ���������
��1��N2 ��g��+3H2 ��g��![]() 2NH3��g����H=��Ӧ����ܺ�-��������ܺ�=945.8kJ��mol��1+3��435.9kJ��mol��1-6��390.8kJ��mol��1=-91.3kJ��mol��1����Ӧ�Ƿ��ȷ�Ӧ����Ӧ���������>���������������
2NH3��g����H=��Ӧ����ܺ�-��������ܺ�=945.8kJ��mol��1+3��435.9kJ��mol��1-6��390.8kJ��mol��1=-91.3kJ��mol��1����Ӧ�Ƿ��ȷ�Ӧ����Ӧ���������>���������������
��2����a����Ӧǰ��������ȣ�����������ѹǿ���䣬��˵���ﵽƽ��״̬����a��ȷ��
b��v(H2)����v(H2)������˵���ﵽƽ��״̬����b��ȷ��
c��N2��H2��Ũ����� ������˵���ﵽƽ��״̬����c����
d�� 2 mol NH3���ɵ�ͬʱ��3 moH��H�����ѣ���Ϊ����Ӧ������˵���Ƿ�ﵽƽ��״̬����d����
e��NH3��Ũ�Ȳ��ٸı�,��˵���ﵽƽ��״̬,��e��ȷ��
��ѡabe��
����2L�ܱ������м���2molN2��6mol H2�����ʵ��Ĵ��������£�������Ӧ N2 ��g��+3H2 ��g��![]() 2NH3��g����10min��ﵽƽ�⣬��ʱʣ��4.5mol H2����
2NH3��g����10min��ﵽƽ�⣬��ʱʣ��4.5mol H2����
N2 ��g��+3H2 ��g��![]() 2NH3��g��
2NH3��g��
��ʼ����mol��L��1��1 3 0
ת������mol��L��1��0.25 0.75 0.5
ƽ�⣺��mol��L��1��0.75 2.25 0.5
0~10min�ڵ�ƽ����Ӧ����v��H2�� ��0.75mol/L��10min=0.075mol��L��1��min-1��
ƽ��ʱ��NH3��Ũ����0.5mol��L��1��
ƽ��ʱ��N2 �ĵ����ʵ���0.75mol��L��1��2L=1.5 mol.
��1����ͼ����Կ�������Ӧ��X��Y�����ʵ�����С��Z�����ʵ������࣬��X��YΪ��Ӧ�ZΪ���������n��X������n��Y������n��Z��=0.3mol��0.1mol��0.2mol=3��1��2����Ӧ�Ļ�ѧ����ʽΪ��3X+Y![]() 2Z��
2Z��
��2����Ӧ��ʼ��
��3��A�������¶ȣ����ʼӿ죬����ȷ��
B����С����X�����ʵ��������ʼ������ʴ���
C����Сѹǿ�����ʼ������ʴ���
D����������Z�����ʵ��������ʼӿ죬����ȷ��
E������ij�ִ��������ʼӿ죬����ȷ��
F����С�ݻ���Ũ���������ʼӿ죬����ȷ��
G��ʹ��Ч�ʸ��ߵĴ��������ʼӿ죬����ȷ��
��ѡADEFG��