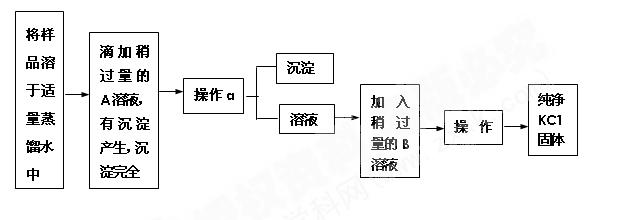
ĚâÄżÄÚČÝ
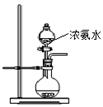
(8·Ö)ÓĂČçĎÂÍĽËůĘľ(ĽĐłÖŇÇĆ÷ʡÂÔ)µÄ×°ÖĂ˝řĐĐʵŃ飬˝«ŇşĚĺAÖđµÎĽÓČëµ˝ąĚĚĺBÖĐŁ¬»Ř´đĎÂÁĐÎĘĚ⣺
˘ŮĎÂÍĽÖĐD×°ÖĂÔÚʵŃéÖеÄ×÷ÓĂĘÇ__________________________________________Ł»
˘ÚČôAΪŨŃÎËᣬBÎŞKMnO4Ł¬CÎŞµí·ŰKIČÜŇşŁ¬ĐýżŞEşóŁ¬CÖеÄĎÖĎóÎŞ____________________Ł¬·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝ĘÇ______________________________________Ł»
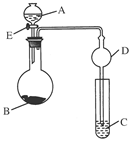
˘ŰČôAΪŨÁňËá(70%)Ł¬BÎŞNa2SO3Ł¬CÎŞËáĐÔKMnO4ČÜŇşŁ¬ĐýżŞEşóŁ¬CÖеÄĎÖĎóÎŞ________________________________________________________________________Ł¬
·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝ĘÇ_______________________________________________Ł»
˘ÜČôAÎŞ30%H2O2ČÜŇşŁ¬BÎŞMnO2Ł»CÎŞH2S±ĄşÍČÜŇşŁ¬ĐýżŞEşóŁ¬CÖеÄĎÖĎóÎŞ________________________________________________________________________Ł¬
·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝ĘÇ__________________________________________________Ł»
˘ÝČôAΪϡŃÎËᣬBÎŞ´óŔíĘŻŁ¬CÎŞNa2SiO3ČÜŇşŁ¬ĐýżŞEşóŁ¬CÖеÄĎÖĎóÎŞ________________Ł¬·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝ĘÇ__________________________________Ł»
˘ŢÉĎÍĽËůĘľ×°ÖĂÓжŕÖÖÓĂÍľŁ¬ÇëľŮŔý(łýĚâÖĐÖ®Íâ)ŁşAÎŞ________Ł¬BÎŞ________Ł¬CÖĐʢ________Ł¬ĐýżŞEşóŁ¬CÖеÄĎÖĎóÎŞ________________Ł¬·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝ĘÇ________________________________________________________________________ˇŁ
˘ŮĎÂÍĽÖĐD×°ÖĂÔÚʵŃéÖеÄ×÷ÓĂĘÇ__________________________________________Ł»
˘ÚČôAΪŨŃÎËᣬBÎŞKMnO4Ł¬CÎŞµí·ŰKIČÜŇşŁ¬ĐýżŞEşóŁ¬CÖеÄĎÖĎóÎŞ____________________Ł¬·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝ĘÇ______________________________________Ł»

˘ŰČôAΪŨÁňËá(70%)Ł¬BÎŞNa2SO3Ł¬CÎŞËáĐÔKMnO4ČÜŇşŁ¬ĐýżŞEşóŁ¬CÖеÄĎÖĎóÎŞ________________________________________________________________________Ł¬
·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝ĘÇ_______________________________________________Ł»
˘ÜČôAÎŞ30%H2O2ČÜŇşŁ¬BÎŞMnO2Ł»CÎŞH2S±ĄşÍČÜŇşŁ¬ĐýżŞEşóŁ¬CÖеÄĎÖĎóÎŞ________________________________________________________________________Ł¬
·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝ĘÇ__________________________________________________Ł»
˘ÝČôAΪϡŃÎËᣬBÎŞ´óŔíĘŻŁ¬CÎŞNa2SiO3ČÜŇşŁ¬ĐýżŞEşóŁ¬CÖеÄĎÖĎóÎŞ________________Ł¬·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝ĘÇ__________________________________Ł»
˘ŢÉĎÍĽËůĘľ×°ÖĂÓжŕÖÖÓĂÍľŁ¬ÇëľŮŔý(łýĚâÖĐÖ®Íâ)ŁşAÎŞ________Ł¬BÎŞ________Ł¬CÖĐʢ________Ł¬ĐýżŞEşóŁ¬CÖеÄĎÖĎóÎŞ________________Ł¬·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝ĘÇ________________________________________________________________________ˇŁ
˘Ů·ŔÖąČÜŇşµąÎü'˘ÚČÜŇşĎȱäŔ¶şóÍĘÉ«'2KIŁ«Cl2===2KClŁ«I2ˇ˘5Cl2Ł«I2Ł«6H2O===2HIO3Ł«10HCl'˘Ű¸ßĂĚËáĽŘČÜŇşµÄ×ĎşěÉ«ÍĘČĄ'5SO2Ł«2H2OŁ«2KMnO4===K2SO4Ł«2MnSO4Ł«2H2SO4'˘ÜČÜŇşÖвúÉúµ»ĆÉ«ąĚĚĺO2Ł«2H2S===
2SˇýŁ«2H2O'˘Ý˛úÉú°×É«˝ş×´łÁµí'CO2Ł«H2OŁ«Na2SiO3===Na2CO3Ł«H2SiO3ˇý'˘ŢŨ°±Ë®'ÉúĘŻ»Ň'ĎőËáŇřČÜŇşşÍĆĎĚŃĚÇČÜŇş'ĘԹܱÚÉĎłöĎÖŇřľµˇˇCH2OH(CHOH)4CHOŁ«
2Ag(NH3)2OHCH2OH(CHOH)4COOHŁ«2 AgˇýŁ«4NH3ˇüŁ«H2O
2SˇýŁ«2H2O'˘Ý˛úÉú°×É«˝ş×´łÁµí'CO2Ł«H2OŁ«Na2SiO3===Na2CO3Ł«H2SiO3ˇý'˘ŢŨ°±Ë®'ÉúĘŻ»Ň'ĎőËáŇřČÜŇşşÍĆĎĚŃĚÇČÜŇş'ĘԹܱÚÉĎłöĎÖŇřľµˇˇCH2OH(CHOH)4CHOŁ«
2Ag(NH3)2OHCH2OH(CHOH)4COOHŁ«2 AgˇýŁ«4NH3ˇüŁ«H2O
ͬһװÖĂŁ¬¶ŕÖÖÓĂÍľŁ¬żĽ˛éѧÉúÁé»îÔËÓĂ֪ʶµÄÄÜÁ¦ˇ˘żŞÍŘѧÉúĘÓŇ°ˇŁ

Á·Ď°˛áϵÁĐ´đ°¸
ĎŕąŘĚâÄż
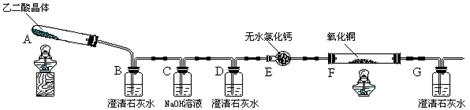
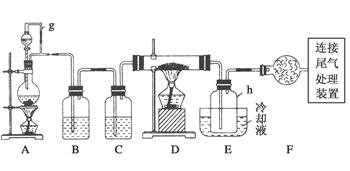
 CaCl2ČÜŇşÖĐͨČëNH3şÍCO2Ł¬żÉŇÔÖƵĂÄÉĂ׼¶ĚĽËá¸ĆŁ¨ÁŁ×ÓÖ±ľ¶ÔÚ1~100nmÖ®ĽäŁ©ˇŁĎÂÍĽËůĘľA~EΪʵŃéĘŇłŁĽűµÄŇÇĆ÷×°ÖĂŁ¨˛ż·ÖąĚ¶¨ĽĐłÖ×°ÖĂÂÔČĄŁ©Ł¬Çë¸ůľÝŇŞÇó»Ř´đÎĘĚ⡣
CaCl2ČÜŇşÖĐͨČëNH3şÍCO2Ł¬żÉŇÔÖƵĂÄÉĂ׼¶ĚĽËá¸ĆŁ¨ÁŁ×ÓÖ±ľ¶ÔÚ1~100nmÖ®ĽäŁ©ˇŁĎÂÍĽËůĘľA~EΪʵŃéĘŇłŁĽűµÄŇÇĆ÷×°ÖĂŁ¨˛ż·ÖąĚ¶¨ĽĐłÖ×°ÖĂÂÔČĄŁ©Ł¬Çë¸ůľÝŇŞÇó»Ř´đÎĘĚ⡣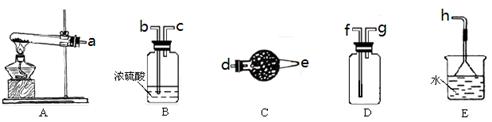

 ÖĂÓĂ´úşĹ±íĘľ)Łş
ÖĂÓĂ´úşĹ±íĘľ)Łş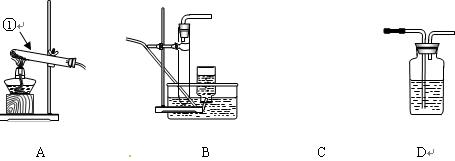
 ĘŇÖƶţŃő»ŻĚĽĘ±Ł¬Ó¦ŃˇÓĂ
ĘŇÖƶţŃő»ŻĚĽĘ±Ł¬Ó¦ŃˇÓĂ µÄ·˘Éú×°ÖĂĘÇ__ __Ł»ĽěŃé¶ţŃő»ŻĚĽĆřĚĺżÉѡÓĂD×°
µÄ·˘Éú×°ÖĂĘÇ__ __Ł»ĽěŃé¶ţŃő»ŻĚĽĆřĚĺżÉѡÓĂD×° ÖĂŁ¬Ćä×°ÖĂÖĐʢ·ĹµÄĘÔĽÁŇ»°ăĘÇ__ __ˇŁ
ÖĂŁ¬Ćä×°ÖĂÖĐʢ·ĹµÄĘÔĽÁŇ»°ăĘÇ__ __ˇŁ