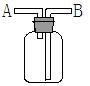
��Ŀ����
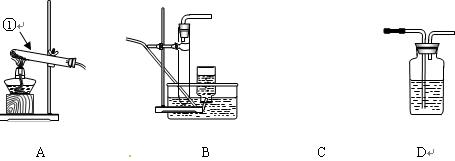
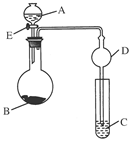
��������ʵ��װ��ͼ�ش�(װ ���ô��ű�ʾ)��
���ô��ű�ʾ)��
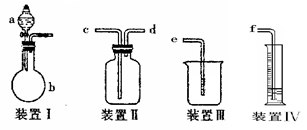
(1)д�����Ϊ�ٵ���������___ _��
(2)ʵ���Ҽ��ȸ������������ʱ��Ӧѡ�õķ���װ����__ __��ʵ�� ���ƶ�����̼ʱ��Ӧѡ��
���ƶ�����̼ʱ��Ӧѡ�� �ķ���װ����__ __�����������̼�����ѡ��Dװ
�ķ���װ����__ __�����������̼�����ѡ��Dװ �ã���װ����ʢ�ŵ��Լ�һ����__ __��
�ã���װ����ʢ�ŵ��Լ�һ����__ __��
(3)ʵ���Ҽ��ȸ��������ȡ������������Ҫ�������裺�ټ��Ȣڰ�ҩƷװ���Թܺ�̶�������̨�Ϣۼ��װ�õ������Ԣ�Ϩ��ƾ��Ƣ�����ˮ���ռ������ˮ����ȡ�����ܡ���ȷ�IJ���˳����(д���)___ _��
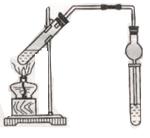
 ���ô��ű�ʾ)��
���ô��ű�ʾ)��
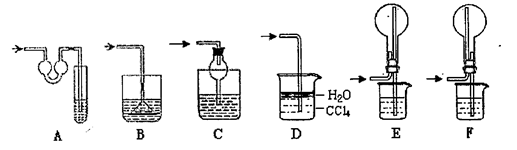
(1)д�����Ϊ�ٵ���������___ _��
(2)ʵ���Ҽ��ȸ������������ʱ��Ӧѡ�õķ���װ����__ __��ʵ��
 ���ƶ�����̼ʱ��Ӧѡ��
���ƶ�����̼ʱ��Ӧѡ�� �ķ���װ����__ __�����������̼�����ѡ��Dװ
�ķ���װ����__ __�����������̼�����ѡ��Dװ �ã���װ����ʢ�ŵ��Լ�һ����__ __��
�ã���װ����ʢ�ŵ��Լ�һ����__ __��(3)ʵ���Ҽ��ȸ��������ȡ������������Ҫ�������裺�ټ��Ȣڰ�ҩƷװ���Թܺ�̶�������̨�Ϣۼ��װ�õ������Ԣ�Ϩ��ƾ��Ƣ�����ˮ���ռ������ˮ����ȡ�����ܡ���ȷ�IJ���˳����(д���)___ _��
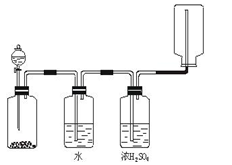
��1���Թ�
��2��A C �����ʯ��ˮ
��3���ۢڢ٢ݢޢ�
��2��A C �����ʯ��ˮ
��3���ۢڢ٢ݢޢ�
��

��ϰ��ϵ�д�
�����Ŀ
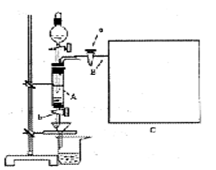
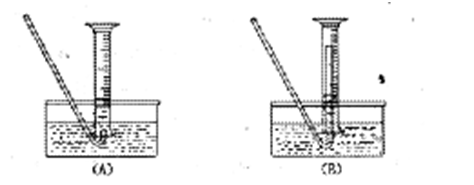
 �����
�����

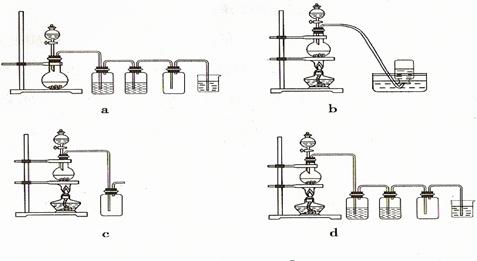

 ��һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ��� ��
��һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ��� �� �����С�������� ��ζ��
�����С�������� ��ζ��