��Ŀ����
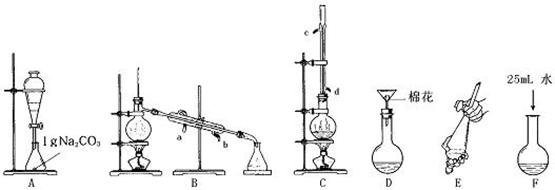
ʵ��������500mL 0.1mol/L Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡʯ��壨ʮˮ̼���ƣ�________g��
��2������Na2CO3��Һʱ��Ҫʹ�õ���Ҫ������������ƽ���ձ���Կ�ס�________��________��________��
��3����ʵ�����������������Һ��Ũ����ƫ�ߣ�ƫ�ͻ��Dz��䣿
�ټ�ˮʱ�����̶��ߣ����õι�����ֱ���̶���________��
�����ǽ�ϴ��Һ��������ƿ________��
������ƿ�ڱڸ���ˮ���δ���ﴦ��________��
�ܶ��ݺ�ҡ�ȣ�Һ����ڿ̶���________��
��1��Ӧ��������ƽ��ȡʯ��壨ʮˮ̼���ƣ�________g��
��2������Na2CO3��Һʱ��Ҫʹ�õ���Ҫ������������ƽ���ձ���Կ�ס�________��________��________��
��3����ʵ�����������������Һ��Ũ����ƫ�ߣ�ƫ�ͻ��Dz��䣿
�ټ�ˮʱ�����̶��ߣ����õι�����ֱ���̶���________��
�����ǽ�ϴ��Һ��������ƿ________��
������ƿ�ڱڸ���ˮ���δ���ﴦ��________��
�ܶ��ݺ�ҡ�ȣ�Һ����ڿ̶���________��
��1��14.3��2��500mL����ƿ������������ͷ�ιܣ�3��ƫ�͢�ƫ�͢۲���ܲ���
��

��ϰ��ϵ�д�
�����Ŀ
 G H I
G H I
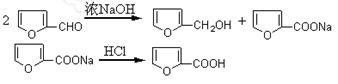
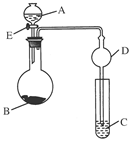
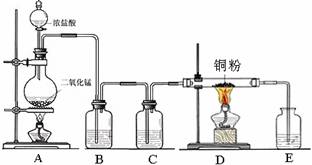
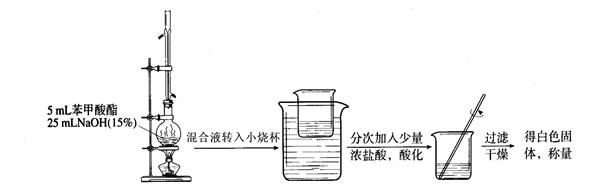
 ��һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ��� ��
��һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ��� �� �����С�������� ��ζ��
�����С�������� ��ζ��