��Ŀ����
(8��)��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ��������֮�������ͼ��ʾ��ת����ϵ��

(1)���A��B��C��D����10���ӵ�������д����A�Ľṹʽ________��D�ĵ���ʽ________��
(2)���A��C��18���ӵ�����B��D��10���ӵ�������д����
��A��B����Һ�з�Ӧ�����ӷ���ʽ___________________________________
________________________________________________________________________��
�ڸ����������ӷ���ʽ�����ж�C��B������ӵ�������С��(�û�ѧʽ�����ӷ��ű�ʾ)________>________��
(3)��֪��(H2N��NH2)�ͼװ�(CH3��NH2)����18�����ӵķ��ӡ������ºͼװ��Ľṹ�ص㲢�����ܵ�������д�����������ͬ���������л�������Ľṹ��ʽ(����д����)��________________________________________________________________________
________________________________________________________________________��
(1) 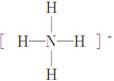 ��H��F��H����������������H
��H��F��H����������������H
(2)��H2S��OH��===HS����H2O��HS����OH��===S2����H2O ��OH����S2��(��HS��)
(3)CH3��CH3��CH3��OH��CH3��F(��������)
��������

���֣���֪A��B��C��D��E����Ԫ�ض���Ԫ�����ڱ���ǰ20��Ԫ�أ�ԭ��������������E�����������Ų�ʽΪ4s2��A��B��C��D����Ԫ����Ԫ�����ڱ��е����λ�����±���ʾ��
| ���� | A | ||||||
| B | C | D |
����������Ϣ���ش��������⣺
(1)Ԫ��C��Ԫ�����ڱ��е�λ���� ���� �壻D�ĵ����Ų�ʽΪ�� ��
(2)A��D���⻯���У��е�ϸߵ��� ��ԭ����
��A��B�������У��뾶��С���� �������ӷ��ţ���
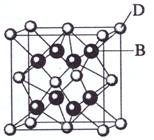
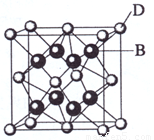
 (3) A��E��������ӻ�����侧��(�������ھ����о��д����Ե���С�ظ���Ԫ)�ṹ����ͼ��ʾ���������á���ʾ��λ�ڸ�������Ķ�������ģ��������á�������ʾ����λ��С���������ģ����û�����ĵ���ʽ�� ��
(3) A��E��������ӻ�����侧��(�������ھ����о��д����Ե���С�ظ���Ԫ)�ṹ����ͼ��ʾ���������á���ʾ��λ�ڸ�������Ķ�������ģ��������á�������ʾ����λ��С���������ģ����û�����ĵ���ʽ�� ��
��4��A��E������ľ���1/8�����Ϊ2.0��10-23cm3����A��E��ɵ����ӻ�������ܶȣ�����ʽ�����㣨�������һλС������_______________________________��
 ��1��д��B�ĵ����Ų�ͼ
��C��Ԫ�ط�����
��B��A�γɵĻ������C ��A�γɵĻ�����е�ߣ���ԭ����
��
��1��д��B�ĵ����Ų�ͼ
��C��Ԫ�ط�����
��B��A�γɵĻ������C ��A�γɵĻ�����е�ߣ���ԭ����
��