��Ŀ����
4��������Ԫ��W��X��Y��Z��ԭ������������������W�������ӵĺ����������X��Y��Zԭ�ӵĺ����ڲ��������ͬ��X��һ�ֺ����ڿ���ʱ����������һЩ������������ҵ�ϲ���Һ̬��������������Y�ĵ��ʣ���Z�����γ�˫ԭ�ӷ��ӣ�������������������˵����ȷ���ǣ�������| A�� | ��������Ԫ�ص�ԭ�Ӱ뾶��СΪW��X��Y��Z | |
| B�� | W��X��Y��Zԭ�ӵĺ����������������ܺ�Ϊ20 | |
| C�� | W��Y���γɼȺ����Թ��ۼ��ֺ��зǼ��Թ��ۼ��Ļ����� | |
| D�� | XW4һ������YW3���ȶ��� |
���� X��һ�ֺ����ڿ���ʱ����������һЩ�����������õ���14C����XΪCԪ�أ���ҵ�ϲ���Һ̬��������������Y�ĵ��ʣ����ǹ�ҵ���������ķ�������YΪNԪ�أ�Z��ԭ����������X��Y���Ҳ����γ�˫ԭ�ӣ����Կ�����ϡ������Ne��X��Y��Z�����ڲ������2��������W��H�����Ԫ�������ɵĵݱ���ɽ����⣮
��� �⣺X��һ�ֺ����ڿ���ʱ����������һЩ�����������õ���14C����XΪCԪ�أ���ҵ�ϲ���Һ̬��������������Y�ĵ��ʣ����ǹ�ҵ���������ķ�������YΪNԪ�أ�Z��ԭ����������X��Y���Ҳ����γ�˫ԭ�ӣ����Կ�����ϡ������Ne��X��Y��Z�����ڲ������2��������W��H����
A��XΪC��YΪN��ͬ����Ԫ�ش�����Ԫ�ص�ԭ�Ӱ뾶��С����ԭ�Ӱ뾶C��N��ZΪNe��ԭ�Ӱ뾶�ⶨ���ݲ�ͬ��һ�㲻������Ԫ�ص�ԭ�Ӱ뾶��Ƚϣ���A����
B��W��X��Y��Zԭ�ӵĺ����������������ܺ�Ϊ1+4+5+8=18����B����
C��W��Y���γ�N2H4�Ļ�����Ⱥ����Թ��ۼ��ֺ��Ǽ��Թ��ۼ�����C��ȷ��
D��WΪHԪ�أ�XΪCԪ�أ�YΪNԪ�أ��ǽ�����Խǿ�����⻯��Խ�ȶ����ǽ����ԣ�N��C����CH4һ������NH3���ȶ��ԣ���D����
��ѡC��
���� ���⿼��ԭ�ӽṹ��Ԫ�������ɵĹ�ϵ����Ŀ�Ѷ��еȣ�����ע����ȷ�ƶ�Ԫ�ص�����Ϊ������Ĺؼ���

| A�� | ��ˮ����ƽ�⣺Br2+H2O=HBr+HBrO��������AgNO3��Һ����Һ��ɫ��dz | |
| B�� | �ڵ�⺬�з�̪����������Һʱ������������Һ����ɫ��� | |
| C�� | ��CO+NO2=CO2+NO��ƽ����ϵ����ѹǿ��ʹ��ɫ���� | |
| D�� | �ϳ�NH3��Ӧ��Ϊ���NH3�IJ��ʣ�������Ӧ��ȡ��Խϵ��¶ȵĴ�ʩ |
| A�� | c��d��a��b | B�� | a��b��d��c | C�� | b��a��d��c | D�� | b��a��c��d |
| A�� |  | B�� | 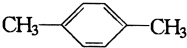 | ||
| C�� |  �������飩 �������飩 | D�� | 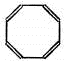 |
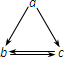 �±����и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������
�±����и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������| ѡ��\���� | a | b | c |
| A | Fe | FeCl2 | FeCl3 |
| B | NaSiO3 | Si | SiO2 |
| C | C | CO | CO2 |
| D | S | SO2 | SO3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | CH3CH2CH2CH3��CH3CH��CH3��2 | B�� | CH2�TC��CH3��2��CH3CH�TCHCH3 | ||
| C�� | CH3CH2OH��CH3OCH3 | D�� | CH3CH2CH2COOH��CH3COOCH2CH3 |
| A�� | �� | B�� | �� | C�� | �� | D�� | �� |
| A�� | ��H2O2+Cl2�T2HCl+O2��Ӧ�У�ÿ����32 g��������ת��4NA������ | |
| B�� | 17.4 gij���ף���ͼ���к�P-S����ĿΪ0.6NA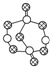 | |
| C�� | 1 mol��8��̼ԭ�ӵ�ij�����ӣ�����γ�7 mol̼̼���� | |
| D�� | ��״���£�22.4 L SO2��O2������壬����2NA����ԭ�� |