��Ŀ����
����Ŀ��ijͬѧ̽�� Cu �� NO �ķ�Ӧ���������ϣ���Cu �� NO ��Ӧ������ CuO �� N2�������������£�NO �� NO2�C������ MnO4�C��Ӧ���� NO3�C�� Mn2+
(1)ʵ��������Cu��ϡ HNO3 �Ʊ� NO��д����Ӧ�Ļ�ѧ����ʽ____��
(2)ѡ����ͼ��ʾװ�����Cu��NO��ʵ�顣(�г�װ����) ʵ�鿪ʼǰ����װ����ͨ��һ��ʱ���N2���ش��������⣺
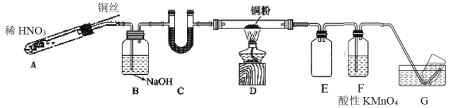
��ʹ��ͭ˿���ŵ���____װ�� E ������Ϊ____��
��װ�� C ��ʢ�ŵ�ҩƷ������____��
��װ�� D �е�������____��װ�� F �з�Ӧ�����ӷ���ʽ��____��
(3)�ⶨNaNO2 �� NaNO3�����Һ��NaNO2��Ũ�ȡ�ȡ 25.00mL�����Һ����ƿ�У���0.1000mol��L��1 ���� KMnO4 ��Һ���еζ���ʵ���������������ʾ��
����� | 1 | 2 | 3 | 4 |
���� KMnO4 ��Һ���/mL | 20.90 | 20.12 | 20.00 | 19.88 |
�ٵ�һ��ʵ�����ݳ����쳣����������쳣��ԭ�������____(����ĸ����)��
a.��ƿϴ����δ����
b.��ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
c.��Ϊû��һֱ�۲���ƿ����Һ��ɫ�仯���жϵζ��յ�ʱ��Һ�ѱ��d.���� KMnO4 ��Һ�к��������������Լ�e.��ƿϴ�����ô���Һ��ϴ
������ KMnO4��Һ�ζ�����������Һ�����ӷ���ʽΪ____��
��NaNO2�����ʵ���Ũ��Ϊ____
���𰸡�3Cu +8HNO3 =3 Cu(NO3)2 + 2NO�� + 4H2O ���Կ��Ʒ�Ӧ�ķ�����ֹͣ ��ȫƿ���������� CaCl2��P2O5 ��ɫ��ĩ��� 5NO +3MnO4-+4H+= 5NO3-+ 3 Mn2++2H2O bce 6H��+2MnO4-+5NO2-=2Mn2++5NO3-+3H2O 0.2mol/L
��������
(2)װ��A����ϡ�����ͭ������Ӧ����NO����װ���ڿ����к���������NO�Ჿ������ΪNO2������װ��B��NaOH��Һ��ȥNO2��NO�پ�װ��C�������D����Cu���ڼ��������·�����Ӧ����CuO �� N2��δ��Ӧ��NO������KMnO4��Һ���գ�������ˮ���ռ����ɵ�N2��
(3) �ٷ�������������V(��)��Ӱ�죬�Դ��ж�Ũ�ȵ���
�ڸ÷�Ӧ������������ӱ�����Ϊ��������ӣ�����������ӱ���ԭΪ�����ӣ�
�۸������ĵ�KMnO4�����ʵ�������Ϸ�Ӧԭ������μӷ�Ӧ���������Ƶ����ʵ�����Ȼ����NaNO2�����ʵ���Ũ�ȡ�
(1) Cu��ϡ HNO3 ��Ӧ����NO��ͬʱ���õ�����ͭ��ˮ��������Ӧ�Ļ�ѧ����ʽΪ3Cu +8HNO3 =3 Cu(NO3)2 + 2NO�� + 4H2O��
(2) �ٿ�ͨ�������ƶ�ͭ˿���Ʒ�Ӧ�Ƿ���У�����ʹ��ͭ˿���ŵ��ǿ��Կ��Ʒ�Ӧ�ķ�����ֹͣ����NO���ױ�����KMnO4��Һ���գ��ײ�������������װ�� E ������Ϊ��ȫƿ��
��װ�� C �������Ǹ���NO��Ӧѡ�����Ի����Թ�����������ʢ�ŵ�ҩƷ������CaCl2��P2O5��
���ڼ��������£�Cu �� NO ��Ӧ������ CuO �� N2����װ�� D �е������Ǻ�ɫ��ĩ��ڣ����������£�NO�� MnO4�C��Ӧ���� NO3�C�� Mn2+����װ�� F �з�Ӧ�����ӷ���ʽ��5NO +3MnO4-+4H+= 5NO3-+ 3 Mn2++2H2O��
(3) �ٵ�һ��ʵ��������Ⱥ�����ʵ����������ƫ��
a����ƿϴ����δ�������Һ�����ʵ����ʵ������䣬�Ա�Һ�������Ӱ�죬��a����
b����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ����Һ��Ũ��ƫС����ɱ�Һ�����ƫ��b��ȷ��
c����Ϊû��һֱ�۲���ƿ����Һ��ɫ�仯���жϵζ��յ�ʱ��Һ�ѱ�죬��μӱ�Һ����������Һ�����ƫ��c��ȷ��
d������ KMnO4 ��Һ�к��������������Լ������ĵı�Һ�����ƫС����d����
e����ƿϴ�����ô���Һ��ϴ����������ʵ����ʵ���ƫ���µζ�ʱ���ĵı�Һ���ƫ��e��ȷ��
�ʴ�Ϊbce��
�ڸ÷�Ӧ������������ӱ�����Ϊ��������ӣ�����������ӱ���ԭΪ�����ӣ����ӷ���ʽΪ6H��+2MnO4-+5NO2-=2Mn2++5NO3-+3H2O��
�۵�һ��ʵ����������ƫ����ȥ��ȡ������ʵ�����ݵ�ƽ��ֵΪ![]() =20.00�������ĸ�����ص����ʵ����ǣ�0.1000mol/L��0.02L=0.002mol������ݷ���ʽ5NO2-+2MnO4-+6H+=5NO3-+2Mn2++3H2O��֪���������Ƶ����ʵ����ǣ�0.002mol��
=20.00�������ĸ�����ص����ʵ����ǣ�0.1000mol/L��0.02L=0.002mol������ݷ���ʽ5NO2-+2MnO4-+6H+=5NO3-+2Mn2++3H2O��֪���������Ƶ����ʵ����ǣ�0.002mol��![]() =0.005mol������Һ���������Ƶ����ʵ���Ũ��Ϊ
=0.005mol������Һ���������Ƶ����ʵ���Ũ��Ϊ![]() =0.2mol/L��
=0.2mol/L��

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�