��Ŀ����
ij�о���ѧϰС��Ϊ�ⶨNH3�����е�����ԭ�Ӹ����ȣ��������ʵ�����̣�
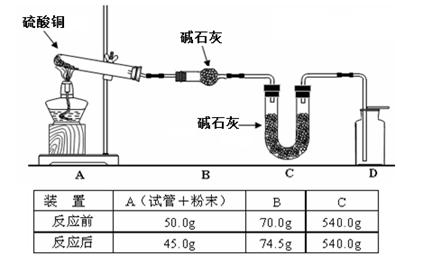
ʵ��ʱ�������Ƶõİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ�ã�������������ͭ��
��ͼA��B��CΪ��С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��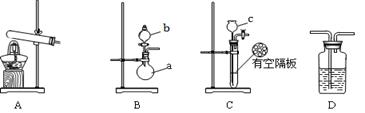
��ش��������⣺
��1��д�����������ƣ�a ��b ��
��2��Ӳ�ʲ������з����ķ�Ӧ����ʽ�� ������Ӧ������Ӳ�ʲ����ܵ������� ��
��3�����ж���ȡ���������õ���װ�ã����±�������Ϊ���е�װ������д��Ӧ��ʵ��ҩƷ��д����ѧʽ����
| װ�� | ʵ��ҩƷ |
| A | |
| B | b�� a�� |
| C | c�� ���壺 |
��4��ʵ��ʱϴ��ƿD�е�Ũ������� �ԣ���С��ʵ���ã�ϴ��ǰװ��D������Ϊm1g��ϴ����װ��D������Ϊm2g�����ɵĵ����ڱ�״���µ����ΪV L�����ݸ�С�����NH3�����е������ԭ�Ӹ����ȵı���ʽ����Ԥ�Ƹý��������ֵ��ȣ� ��
A����ʵ����Χ����ֵ�ӽ�����ֵ B����ֵƫ�� C����ֵƫ��
��1��Բ����ƿ��2�֣�����Һ©����2�֣�
��2��2NH3��3CuO 3Cu��N2��3H2O��2�֣�����ɫ�����ɺ�ɫ��2�֣�
3Cu��N2��3H2O��2�֣�����ɫ�����ɺ�ɫ��2�֣�
��3����ÿ�ֿ���װ�ø�2�֣���4�֣�װ�� ʵ��ҩƷ A NH4Cl��Ca(OH)2 B b��NH3·H2O(aq) a��CaO�� NaOH��CaO��NaOH
��4����ˮ���ᣨ2�֣��� C ��2�֣�
���������������1����ͼ�п�֪����a������Ϊ��Բ����ƿ��bΪ��Һ©����
��2����������������ȡ�õ��İ��������ͨ����Ӳ�ʲ������У����ڼ�������CuO�����˷�Ӧ������CuO�������ԣ������л�ԭ�ԣ����Զ��߷���������ԭ��Ӧ������ʽΪ2NH3��3CuO 3Cu��N2��3H2O���ɴ˿�֪��ͭ�������ɣ����Կɹ۲쵽�����Ǻ�ɫ�������˺�ɫ���ʡ�
3Cu��N2��3H2O���ɴ˿�֪��ͭ�������ɣ����Կɹ۲쵽�����Ǻ�ɫ�������˺�ɫ���ʡ�
��3��ͼ��A��B��CΪ��С����ȡ����ʱ�����õ���װ�ã����Ը��ݰ����Ʊ���ԭ���������ǹ�-�̼����ͣ�Ҳ�����ǹ�-Һ�������ͣ����Ը���װ�����Ϳ���ѡ����Ӧʵ��ҩƷΪ��װ�� ʵ��ҩƷ A NH4Cl��Ca(OH)2 B b��NH3·H2O(aq) a��CaO�� NaOH��CaO��NaOH
��4������ϴ��ƿD�е�Ũ����������˷�Ӧ���ɵ�ˮ���������˹����İ��������Ա��ֵ���Ũ�������ˮ�Ժ����ԣ�����ΪŨ����������ˮ�Ͱ��������Ը��ݸü��㹫ʽ����ʹ�����H����ƫ�ߣ���N:H������С��С������ֵ��
���㣺���⿼��������ð����Ļ�ԭ�Խ��е�̽��ʵ�飬�������˰�����ʵ�����Ʊ���Ũ�������ʡ�

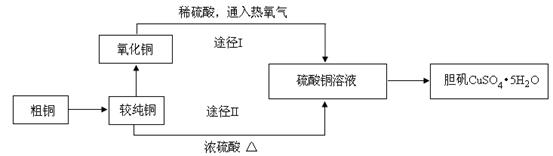
(14��)������һ����Ҫ�Ļ���ԭ�ϣ��㷺����ҩ���������߷��Ӻϳɵȹ�ҵ�����ᾧ�����ȵ�100��ʱʧȥ�ᾧˮ����Ϊ��ˮ���ᡣijѧϰС���ͬѧ���Ը�����Ϊԭ����ˮ�⡪������ˮ��ѭ��������ȡ���ᡣ
|
 �����������Ϣ�ش��������⣺
�����������Ϣ�ش��������⣺��1��ͼʾ�٢ڵ�������ˮ�����������ͼ1��װ���н��еģ�ָ��װ��A������ ��
��2��ͼʾ�٢ڵ�������ˮ������У���������������Ӧ��ʱ�����������ͬ������£��ı䷴Ӧ�¶��Կ��췴Ӧ�¶ȶԲ������ʵ�Ӱ�죬�������ͼ2��ʾ����ѡ����ѵķ�Ӧ�¶�Ϊ ��Ϊ�˴ﵽͼ2��ʾ���¶ȣ�ѡ��ͼ1��ˮԡ���ȣ����ŵ��� ��
��3����ͼʾ�ۢ��еIJ����漰�����ˣ�ϴ�ӡ���������й�˵����ȷ���� ��
A.��ʵ������У�ͨ��������ȴ������Һ�����Եõ��ϴ�ľ�����������ڳ���
B.��ϴ�ӳ���ʱ��Ӧ��Сˮ��ͷ��ʹϴ�Ӽ�����ͨ��������
C.Ϊ�˼���ϴ���Ƿ���ȫ��Ӧ��������ƿ�밲ȫƿ֮����Ƥ�ܣ�������ƿ�Ͽڵ���������Һ���Թ��н������ʵ�顣
D.Ϊ�˵õ�����ľ��壬����ѡ����������ֱ�Ӽ��ȣ����ڸ���������ȴ��
��4��Ҫ�ⶨ���ᾧ�壨H2C2O4��2H2O���Ĵ��ȣ���ȡ7.200g�Ʊ��IJ��ᾧ����������ˮ���250mL��Һ��ȡ25.00mL������Һ����ƿ�У���0.1000mol/L���Ը��������Һ�ζ�
��5H2C2O4+2MnO4��+6H+��2Mn2++10CO2��+8H2O����
��ȡ25.00mL������Һ�������� ��
���ڲ��ᴿ�Ȳⶨ��ʵ������У�����˵����ȷ���ǣ� ��
A.��ϴ�ζ���ʱ��Ӧ�ӵζ����Ͽڼ������������Һ��ʹ�ζ����ڱڳ����ϴ
B.��Һ��ȡ������Һʱ���轫���촦��Һ�崵����ƿ����ʹʵ�����ƫ��
C.�ζ�ʱ�������������ڿ�ס���������Ŀ���������������ʹ���ɶ�©����Һ
D.�ζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬��ʹʵ�����ƫ��
���жϵζ��Ѿ��ﵽ�յ�ķ����ǣ� ��
�ܴﵽ�ζ��յ�ʱ�����ĸ��������Һ��20.00mL������ᾧ��Ĵ���Ϊ ��
�⻯�ƹ����ǵ�ɽ�˶�Ա���õ���Դ�ṩ����ij��ȤС����ѡ������װ���Ʊ��⻯�ơ�
�ش��������⣺
��1�����װ��E�����ԵIJ��������� ��
��2����������װ����ȡ�⻯��ʱ��������������˳��Ϊi��___��___��___�� �� �� ��a���������ӿڵ���ĸ��ţ���
��3������������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ����Һ©��������__________________���밴��ȷ��˳���������в���ı�ţ���
| A�����ȷ�Ӧһ��ʱ�� | B���ռ����岢�����䴿�� |
| C���رշ�Һ©������ | D��ֹͣ���ȣ������ȴ |
����ʵ�鲻�ܴﵽԤ��ʵ��Ŀ�ĵ��ǣ� ��
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��ʢ��10��0.1 mol��L��1 AgNO3��Һ���Թ��еμ�0.1 mol��L��1 NaCl��Һ���������г������ɣ��������еμ�0.1 mol��L��1 Na2S��Һ | ֤��AgCl��ת��Ϊ�ܽ�ȸ�С��Ag2S |
| B | ��2 mL�ױ��м���3������KMnO4��Һ������2 mL���м���3������KMnO4��Һ���� | ֤���뱽�������ļ��ױ� ���� |
| C | ��Na2SiO3��Һ��ͨ��CO2 | ֤��̼������Աȹ���ǿ |
| D | �������Һ�м���ϡ���ᣬˮԡ���ȣ�һ��ʱ����ټ������Ƶ�������ͭ����Һ������ | ��֤������ˮ�� |

 FeSO4+H2��
FeSO4+H2�� +5C2
+5C2 +16H+
+16H+

