��Ŀ����
����Ŀ������ȩ��Ũ����������Һ�з���Cannizzaro��Ӧ����Ӧ����ʽ���£��������Ʊ�������ͱ��״���
2![]() +KOH
+KOH![]()
![]() \
\
![]() +HCl
+HCl![]()
��֪��
����������ˮ�����л������õ��ܼ����е�34.6�棬���ӷ����ڿ����еķе�160�档
�ڱ�������ˮ�е��ܽ��0.17g(25��),0.95g(50��),6.8g(95��)
ʵ�鲽�裺
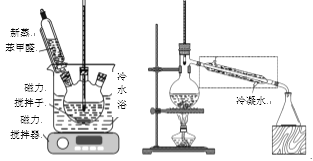
����50mL����ƿ�м���4.5g KOH��4.5mLˮ����װ��������ˮԡ�У���������������������5mL ��������ȩ(�ܶ�1.04g/mL)��ʹ��Ӧ���ֻ��(������ͼ)������Ϊ��ɫ��״�����24h���ϡ�
����Ӧ���������������ˮ����ʹ����ȫ�ܽ����_____(��װ������)�У���10mL ������ȡ3�Σ��ϲ�������ȡҺ����������3mL�������������ơ�5mL 10% Na2CO3��Һ��5mLˮϴ�ӣ��ֳ������Ѳ�����ˮNa2CO3���
�۸�����������Һ��ˮԡ����ȥ���ѣ�Ȼ���ʵ���������װ��(������ͼ)���������������ռ�198�桫204��ı��״���֣�����Ϊ2.16g��
��������ȡ���ˮ��Һ(ˮ��)����Ũ�����ữ�������ȴ��ʹ��������ȫ���������ˣ��ֲ�Ʒ��ˮ�ؽᾧ�õ������ᣬ������2g��
(1)���Ͻ���Ȼ�����24h��Ŀ����____________________________________
(2)����ں��ߴ�װ��������___________________________
(3)��ȡҺ��ϴ����3�Σ�����10% ̼������Һ����ȥ��������_______(�ѧʽ)

(4)ʹ��ˮԡ��ȥ���ѵ��ŵ���_____________
(5)��ȥ���Ѻ��ʵ��ĵ�������װ�ã�Ӧ������ͼ���߿��е�װ�û�Ϊ________��
(6)�������ؽᾧʱ��������ˮϴ�ӹ��壬��Ŀ����______���������ؽᾧʱ����IJ���������__________________
���ձ� ���Թ� ����ƿ �ܾƾ��� ����Ͳ �̾�����©�� �߲�����
(7)��ʵ����Cannizzaro��Ӧ��ת������_____%(����1λС��)��ͨ������ʵ���б�����IJ��ʻ�ȱ��״����ͣ�������ij��ʵ���в�δ����ƿ�������±�����IJ�������ƫ�ߣ��������ܵ�ԭ����__________��
���𰸡���Һ©�� ʹ��Ӧ���Ͼ��ȣ���ַ�Ӧ ��Һ©�� NaHSO3 ˮԡ���ȿ��ṩ�㶨���¶ȣ��ﵽ���Ⱦ����ұ��ڿ����¶ȵ�Ŀ�� c ���ٱ�������ܽ�ȴ�������ʧ �٢ܢݢޢ� 81.6% ԭ���еı���ȩ�����������������ɱ����ᣬ���±�����ĺ�������ƫ��
��������
��ȡ������ʹ�õ������ķ�Һ©����
(1)���Ͻ���Ȼ�����24h����ʹ��Ӧ���Ͼ��ȣ���ַ�Ӧ��
(2)��ȡ�õ��������Ƿ�Һ©����
(3)ʵ����뱥�����������ƣ��ɼ���̼���Ƴ�ȥ���ʴ�Ϊ��NaHSO3��
(4)ˮԡ���ȿ��ṩ�㶨���¶ȣ��ﵽ���Ⱦ����ұ��ڿ����¶ȵ�Ŀ�ģ�
(5)��ȥ���Ѻ��ʵ��ĵ�������װ�ã��ռ�198��-204��ı��״���֣�����ϵ��¶Ƚ����������ɸ��ÿ��������ܣ���˴�Ϊc��
(6)�������ؽᾧʱ��������ˮϴ�ӹ��壬�ɽ��ͱ�������ܽ�ȣ�������ʧ���������ؽᾧ��Ҫ�ձ����ƾ����Լ��������Լ��̾�����©��������������ʹ������������Ǣ٢ܢݢޢߣ�
(7)����5mL��������ȩ(�ܶ�1.04g/mL)�����������Ϊ5.2g�����ʵ���Ϊn=![]() =0.049mol�����ۿ����ɱ����������m=
=0.049mol�����ۿ����ɱ����������m=![]() ��122g/mol=2.989g�����ɱ��״�������Ϊm(���״�)=
��122g/mol=2.989g�����ɱ��״�������Ϊm(���״�)=![]() ��108g/mol=2.646g����Cannizzaro��Ӧ��ת�����ɱ��״����㣬ת����Ϊ
��108g/mol=2.646g����Cannizzaro��Ӧ��ת�����ɱ��״����㣬ת����Ϊ![]() ��100%=81.6%��ԭ���еı���ȩ�ɱ��������ɱ����ᣬʵ���в�δ����ƿ�������±�����IJ�������ƫ�ߡ�
��100%=81.6%��ԭ���еı���ȩ�ɱ��������ɱ����ᣬʵ���в�δ����ƿ�������±�����IJ�������ƫ�ߡ�





