��Ŀ����
19�� ��ѧ��Ԥ�ԣ�ȼ�ϵ�ؽ��� 21���ͻ�õ��ܵ���Ҫ;���������꿪���ļ״�ȼ�ϵ���Dz��ò����缫������е����ӽ���Ĥֻ���� H+ ��ˮ����ͨ�����乤��ԭ����ʾ��ͼ���£�����˵��������ǣ�������
��ѧ��Ԥ�ԣ�ȼ�ϵ�ؽ��� 21���ͻ�õ��ܵ���Ҫ;���������꿪���ļ״�ȼ�ϵ���Dz��ò����缫������е����ӽ���Ĥֻ���� H+ ��ˮ����ͨ�����乤��ԭ����ʾ��ͼ���£�����˵��������ǣ�������| A�� | a �Ǹ�����b ������ | |
| B�� | b ���ĵ缫��Ӧ�� O2+4H++4e-�T2H2O | |
| C�� | �ŵ�����У�������������Ϊ��a��b�����ӽ���Ĥ��a | |
| D�� | ����·��ͨ�� 2 mol �������ĵ� CH3OH Ϊ $\frac{1}{3}$mol |
���� ��ԭ��������ӽ���Ĥֻ�������Ӻ�ˮ����ͨ����˵���������ҺΪ������Һ��ȼ�ϵ���У�ͨ��ȼ�ϵĵ缫Ϊ����������a �Ǹ�����������ʧ���ӷ���������Ӧ����ⷽ��ʽΪCH3OH+H2O-6e-�TCO2+6H+��ͨ���������ĵ缫Ϊ����������b �������������ϵõ��ӷ�����ԭ��Ӧ���缫����ʽΪO2+4H++4e-�T2H2O���ɴ˷������
��� �⣺A��ͨ��ȼ�ϵĵ缫Ϊ����������a �Ǹ�����ͨ���������ĵ缫Ϊ����������b ����������A��ȷ��
B��b �������������ϵõ��ӷ�����ԭ��Ӧ���缫����ʽΪO2+4H++4e-�T2H2O����B��ȷ��
C���ŵ�����У�������������Ϊ��a$\stackrel{����}{��}$b����C����
D���ɵ缫����ʽCH3OH+H2O-6e-�TCO2+6H+��֪������õ�ع���ʱ��·��ͨ��2mol���ӣ������ĵ�CH3OH��Ϊ $\frac{1}{3}$mol����D��ȷ��
��ѡC��
���� ���⿼����ȼ�ϵ�أ��������ӽ���Ĥͨ������ȷ���������Һ������ԣ��ٽ���������Ϸ����ķ�Ӧ�����������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
9�����ж��л���ṹ�����ʵ���������ȷ���ǣ�������
| A�� | ����ͱ�ϩ�Ļ���ﹲ1mol�����ȼ�պ���ȷ�����ɵ�ˮ�����ʵ��� | |
| B�� | ����������Ȳ�ͱ���ϩ�����ȼ�պ���ȷ���������Ƿ���ͬ | |
| C�� | ����屽����һ�ֿռ�ṹ������˵�����в����ڵ�˫������Ľṹ | |
| D�� | ������������Һ��������ֲ���ͺͿ����� |
10��������������뻷����Ⱦ�йص��ǣ�������
�ٱ��� �ڳ�ϫ������ܵ��� �ݳ�����ն���ɽ���� �ߴ��Ӳ� ��ȫ���������ů��
�ٱ��� �ڳ�ϫ������ܵ��� �ݳ�����ն���ɽ���� �ߴ��Ӳ� ��ȫ���������ů��
| A�� | �ۢݢ� | B�� | �ڢۢݢ� | C�� | �٢ۢݢ� | D�� | �ڢۢܢ� |
7��Rԭ��������15�����й���RԪ�ص�˵���У�������ǣ�������
| A�� | R�ǵ������ڵڢ�A���Ԫ�� | |
| B�� | R����������ϼ���+5 | |
| C�� | R���⻯�����ʽ��RH5 | |
| D�� | R������������ˮ�����ˮ��Һ������ |
14���������ӷ���ʽ��һ����ȷ���ǣ�������
| A�� | ��������ϡ���ᷴӦ��FeS+2H+��Fe2++H2S | |
| B�� | ������Һ�еμ��������CO32-+H+��HCO3- | |
| C�� | ������NaHSO4��Ba��OH��2��Һ��Ӧ��Ba2++2OH-+2H++SO42-��BaSO4��+2H2O | |
| D�� | ������SO2����ͨ��Ca��ClO��2��Һ�У�SO2+Ca2++2ClO-+H2O��CaSO3��+2HClO |
4��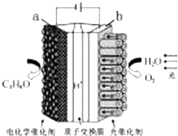 ijģ�⡰�˹���Ҷ���绯ѧʵ��װ����ͼ��ʾ����װ���ܽ�H2O��CO2ת�� ΪO2��ȼ�ϣ�C3H8O��������˵����ȷ���ǣ�������
ijģ�⡰�˹���Ҷ���绯ѧʵ��װ����ͼ��ʾ����װ���ܽ�H2O��CO2ת�� ΪO2��ȼ�ϣ�C3H8O��������˵����ȷ���ǣ�������
 ijģ�⡰�˹���Ҷ���绯ѧʵ��װ����ͼ��ʾ����װ���ܽ�H2O��CO2ת�� ΪO2��ȼ�ϣ�C3H8O��������˵����ȷ���ǣ�������
ijģ�⡰�˹���Ҷ���绯ѧʵ��װ����ͼ��ʾ����װ���ܽ�H2O��CO2ת�� ΪO2��ȼ�ϣ�C3H8O��������˵����ȷ���ǣ�������| A�� | ��װ�ý���ѧ��ת��Ϊ���ܺ͵��� | |
| B�� | ��װ�ù���ʱ��H+��b������a����Ǩ�� | |
| C�� | ÿ����1 mol O2����44 g CO2����ԭ | |
| D�� | a �缫�ķ�ӦΪ��3CO2+18H+-18e-�TC3 H8O+5 H2 O |
13�����и����е����ʱȽϣ���ȷ���ǣ�������
| A�� | ����HClO4��HBrO4��HIO4 | B�� | ����NaOH��Mg��OH��2��Ca��OH��2 | ||
| C�� | �ȶ��ԣ�HI��H2S��HCl | D�� | �����ԣ�Na+��Mg2+��Al3+ |
14������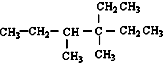 ����ij��ϩ����H2�ӳɺ�IJ������ϩ����˳���칹�������ֵ�ϩ���Ľṹ�����У�������
����ij��ϩ����H2�ӳɺ�IJ������ϩ����˳���칹�������ֵ�ϩ���Ľṹ�����У�������
 ����ij��ϩ����H2�ӳɺ�IJ������ϩ����˳���칹�������ֵ�ϩ���Ľṹ�����У�������
����ij��ϩ����H2�ӳɺ�IJ������ϩ����˳���칹�������ֵ�ϩ���Ľṹ�����У�������| A�� | 3�� | B�� | 4 | C�� | 5�� | D�� | 6�� |