��Ŀ����
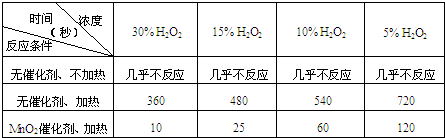
��8�֣��������⣨H2O2����һ����ɫճ��Һ�壬��ˮ��Һ���׳�˫��ˮ���������ԣ�ҽ��������������������о�������H2O2��������ṹ��������ԭ�����ڰ�չ���鱾����ҳֽ�ϣ���ҳֽ��ļн�ԼΪ94�㣬��ԭ������ļз��ϣ�O��H����O��O��֮��Ŀռ�н�ԼΪ97��

��H2O2�ĵ���ʽΪ �� �ƾ��ⶨ��H2O2Ϊ��Ԫ���ᣬ�����Ա�̼��������д�����һ���ĵ��뷽��ʽ_____________��
��H2O2��Һ��һ����������������ﷴӦ����ǿ�ᣬ�÷�Ӧ�����ӷ���ʽΪ �����ڷ���ʽ�ϱ������ת�Ƶķ�������Ŀ��
��Ϊ�����桢���䡢ʹ�õķ��㣬��ҵ�ϲ��á�������������ת��Ϊ��̬�Ĺ�̼���ƾ��壨�仯ѧʽΪ2Na2CO3��3H2O2�����þ������Na2CO3��H2O2��˫�����ʡ�������������ʹ��̼���ƽϿ�ʧЧ���ǣ���ѡ�� ��
A. MnO2 B. H2S C. ϡH2SO4 D. NaHCO3
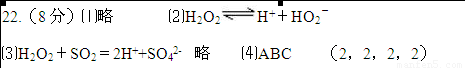
���𰸡�
����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ