��Ŀ����
3���������У���O3 ��1H+��KOH ��2H ��H2O2 ��H2 ��NH4Cl ��3H ��H2SO4 ��MgF2⑪234U⑫O2⑬Cl2⑭Ar⑮Na2O2��1�����������У����ں��ص��Ǣܢ�⑪ ������ţ���ͬ����
��2����Ϊͬ����������Ǣ�⑫��
��3��ֻ���ڹ��ۼ��Ļ������Ǣݢᣬ
��4��ֻ�������Ӽ����Ǣ⣮
��5���ȴ������Ӽ��ִ��ڼ��Թ��ۼ����Ǣۢߣ�
��6���ȴ������Ӽ��ִ��ڷǼ��Թ��ۼ�����⑮��
���� ��1����ʾ����ʱ��������������
��2����ͬ��Ԫ����ɵIJ�ͬ���ʻ�Ϊͬ�������壻��������ͬ������������ͬ��ԭ�ӻ�Ϊͬλ�أ�
��3�����ۻ�������ֻ���ڹ��ۼ���
��4�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ���
��5�����ӻ������У��ɲ�ͬ�ǽ���Ԫ���γɵ������Ӻ��м��Լ���
��6�����ӻ������У�����ͬ�ǽ�����Ԫ���γɵ������Ӻ��зǼ��Լ���
��� �⣺��1����ʾ����ʱ�����������������Тܢ�⑪Ϊ���أ��ʴ�Ϊ���ܢ�⑪��
��2����ͬ��Ԫ����ɵIJ�ͬ���ʻ�Ϊͬ�������壬��⑫����ͬ�������壬�ʴ�Ϊ����⑫��
��3�����ۻ�������ֻ���ڹ��ۼ����ɷǽ���Ԫ�ع��ɣ���γ��⣬�ݢ����ڹ��ۻ�����ʴ�Ϊ���ݢ
��4�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ�������ϣ��ʴ�Ϊ���⣻
��5�����ӻ������У��ɲ�ͬ�ǽ���Ԫ���γɵ������Ӻ��м��Լ����ۢ߷��ϣ��ʴ�Ϊ���ۢߣ�
��6�����ӻ������У�����ͬ�ǽ�����Ԫ���γɵ������Ӻ��зǼ��Լ���⑮���ϣ��ʴ�Ϊ��⑮��
���� ���⿼����ͬλ�ء�ͬ����������жϣ�����������ѧ�����͵��жϣ���ȷ��ظ����ǽ���ؼ���ע�����Ӽ��빲�ۼ���������Ŀ�ѶȲ���

| A�� | ���� | B�� | ������Ƭ���������� | ||
| C�� | �ʵ��������Ũ�� | D�� | ����ϡ���ᣬ����98%Ũ���� |
| A�� | �ӳɷ�Ӧ | B�� | �ۺϷ�Ӧ | C�� | ��ȥ��Ӧ | D�� | ������Ӧ |
| A�� | K+��Cu2+��Cl-��SO42- | B�� | NO3-��Mg2+��S2-��SO42- | ||
| C�� | HCO3-��I-��Ca2+��Cl- | D�� | K+��Ca2+��CO32-��Cl- |
| A�� | ��ϩ | B�� | H2O2 | C�� | ���� | D�� | ���ȼ��� |
| A�� | ú��������Һ�����������仯���̣��ɱ�Ϊ�����Դ | |
| B�� | ά����C���л�ԭ�ԣ������������������� | |
| C�� | ������һ�����ԣ��������������ͷ����� | |
| D�� | ����������ľ�����ά�����ڹ���ͨ�ŵĹ��ά�����������ǽ������� |
| A�� | ͨ��״���£�1g����ȼ������Һ̬ˮʱ�ų�142.9kJ�����������ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ2H2��g��+O2��g��=2H2O��l����H=-571.6kJ/mol | |
| B�� | ��֪H2��g��+F2��g���T2HF��g������H=-270kJ/mol����1mol������1mol������Ӧ����2molҺ̬������ų�������С��270kJ | |
| C�� | 500�桢30MPa�£���0.5mol N2��1.5mol H2�����ܱյ������г�ַ�Ӧ����NH3��g��������19.3kJ�����Ȼ�ѧ����ʽΪN2��g��+3H2��g��$?_{����}^{���¡���ѹ}$2NH3��g������H=-38.6kJ/mol | |
| D�� | ��֪����C��sʯī��+O2��g���TCO2��g������H=-393.5kJ/mol����C��s�����ʯ��+O2��g���TCO2��g������H=-395.0kJ/mol����C��s�����ʯ���TC��s��ʯī������H=-1.5kJ/mol |
| A�� | 0.1 mol/L��0.2 mol/L������Һ��c��H+��֮�� | |
| B�� | 0.1 mol/L H2S��Һ��c��S2-����c��H+��֮�� | |
| C�� | pH=10��Ba��OH��2��Һ��pH=10�İ�ˮ�����ʵ����ʵ���Ũ��֮�� | |
| D�� | pH=3��������pH=3�Ĵ�����Һ��c��SO42-����c��CH3COO-��֮�� |
 �л���A��һ�ֺ������������ʽΪC6H9O2Br����֪�����µ�ת����ϵ��
�л���A��һ�ֺ������������ʽΪC6H9O2Br����֪�����µ�ת����ϵ��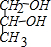 ��HOCH2CH2CH2OH��
��HOCH2CH2CH2OH��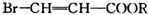 ��R��ʾ����������2�֣�
��R��ʾ����������2�֣�