��Ŀ����
ijС��ͬѧ��һ��Ũ��NaHCO3��Һ���뵽CuSO4��Һ�з��������˳�������ͬѧ��Ϊ������CuCO3;��ͬѧ��Ϊ������CuCO3��Cu(OH)2�Ļ����,�������ʵ��ⶨ������CuCO3������������
(1)���ռ�ͬѧ�Ĺ۵�,������Ӧ�����ӷ���ʽΪ ��
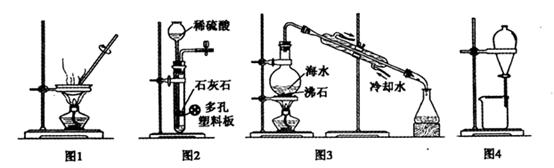
(2)��ͬѧ������ͼ��ʾװ�ý��вⶨ:

�����о����������ǰ,�뽫��������Һ�з��벢�����������������Ϊ���ˡ�ϴ�ӡ����
��װ��E�м�ʯ�ҵ������� ��
��ʵ������������²�������:
a.�ر�K1��K3,��K2��K4,��ַ�Ӧ
b.��K1��K4,�ر�K2��K3,ͨ���������
c.��K1��K3,�ر�K2��K4,ͨ���������
��ȷ��˳���� (��ѡ�����,��ͬ)����δ���в��� ,��ʹ�������ƫ�͡�
����������Ʒ����Ϊm g,װ��D����������n g,�������CuCO3����������Ϊ ��
(3)��ͬѧ��Ϊ������ͨ������CO2����������� ���ⶨ������CuCO3������������
(1)���ռ�ͬѧ�Ĺ۵�,������Ӧ�����ӷ���ʽΪ ��
(2)��ͬѧ������ͼ��ʾװ�ý��вⶨ:

�����о����������ǰ,�뽫��������Һ�з��벢�����������������Ϊ���ˡ�ϴ�ӡ����
��װ��E�м�ʯ�ҵ������� ��
��ʵ������������²�������:
a.�ر�K1��K3,��K2��K4,��ַ�Ӧ
b.��K1��K4,�ر�K2��K3,ͨ���������
c.��K1��K3,�ر�K2��K4,ͨ���������
��ȷ��˳���� (��ѡ�����,��ͬ)����δ���в��� ,��ʹ�������ƫ�͡�
����������Ʒ����Ϊm g,װ��D����������n g,�������CuCO3����������Ϊ ��
(3)��ͬѧ��Ϊ������ͨ������CO2����������� ���ⶨ������CuCO3������������
(1)Cu2+ + 2HC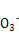 =uCO3��+ CO2��+ H2O
=uCO3��+ CO2��+ H2O
(2)�ڷ�ֹ������CO2��ˮ��������װ��D �� cab b
��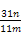 ��100%
��100%
(3)��Ʒ����(����Ʒ������պ������)(����������Ҳ��)
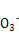 =uCO3��+ CO2��+ H2O
=uCO3��+ CO2��+ H2O(2)�ڷ�ֹ������CO2��ˮ��������װ��D �� cab b
��
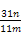 ��100%
��100%(3)��Ʒ����(����Ʒ������պ������)(����������Ҳ��)
(1)����Ĺ۵���ȷ,������̼��ͭ��ͬʱ,��Ȼ������CO2��ˮ,���ӷ���ʽΪCu2+ + 2HC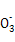
 CuCO3��+CO2��+ H2O��
CuCO3��+CO2��+ H2O��
(2)�����ڿ�����Ҳ����ˮ������CO2,����E�м�ʯ�ҵ������Ƿ�ֹ�����е�CO2��ˮ��������װ��D,����ʵ������
������װ����Ҳ���п���,��������Ҫ�ž�װ���еĿ���,Ȼ���ٷ�Ӧ�����ڷ�Ӧ�����ɵ�����������װ����,��˷�Ӧ������,��ͨ���������Ӧ���ɵ�����ȫ���ų�,�����ȷ�Ĵ�ѡcab�����������b����,��ʹCO2����ƫС,��ʹ�������ƫ�͡�
��D������CO2��,����̼��ͭ��������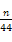 ��124 g,����̼��ͭ������������
��124 g,����̼��ͭ������������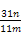 ��100%��
��100%��
(3)��CO2������ɼ���CuCO3�����ʵ���,������ֱ�ӻ��Ӳ����Ʒ��������
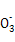
 CuCO3��+CO2��+ H2O��
CuCO3��+CO2��+ H2O��(2)�����ڿ�����Ҳ����ˮ������CO2,����E�м�ʯ�ҵ������Ƿ�ֹ�����е�CO2��ˮ��������װ��D,����ʵ������
������װ����Ҳ���п���,��������Ҫ�ž�װ���еĿ���,Ȼ���ٷ�Ӧ�����ڷ�Ӧ�����ɵ�����������װ����,��˷�Ӧ������,��ͨ���������Ӧ���ɵ�����ȫ���ų�,�����ȷ�Ĵ�ѡcab�����������b����,��ʹCO2����ƫС,��ʹ�������ƫ�͡�
��D������CO2��,����̼��ͭ��������
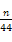 ��124 g,����̼��ͭ������������
��124 g,����̼��ͭ������������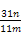 ��100%��
��100%��(3)��CO2������ɼ���CuCO3�����ʵ���,������ֱ�ӻ��Ӳ����Ʒ��������

��ϰ��ϵ�д�
�����Ŀ