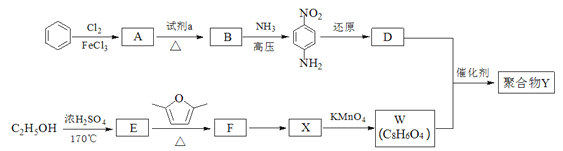
��Ŀ����
����Ŀ����t��Cʱ��AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��CʱAgCl��Ksp=4��10-10������˵������ȷ���ǣ� ��
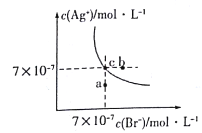
A. ͼ��a���Ӧ����AgBr�IJ�������Һ
B. ��t ��Cʱ��AgBr��KspΪ 4.9��10-13
C. ��AgBr������Һ�м���NaBr���壬��ʹ��Һ��c�㵽b��
D. ��t ��Cʱ��AgCl��s��+Br-��aq��![]() AgBr��s��+C1- ��aq����ƽ�ⳣ��K��816
AgBr��s��+C1- ��aq����ƽ�ⳣ��K��816
���𰸡�C
�����������������A������ͼ���֪����a��ʱQc=c(Ag+)c(Br-)��Ksp������a��ΪAgBr�IJ�������Һ����A��ȷ��B�����ͼ��c���c(Ag+)��c(Br-)��֪�����¶���AgBr��Ksp=7��10-7��7��10-7=4.9��10-13����B��ȷ��C����AgBr������Һ�м���NaBr�����c(Br-)�����ܽ�ƽ�������ƶ���c(Ag+)��С����C����D����ӦAgCl(s)+Br-(aq)AgBr(s)+Cl-(aq)��ƽ�ⳣ��Ϊ��K=![]() =
=![]() ��816����D��ȷ����ѡC��
��816����D��ȷ����ѡC��

��ϰ��ϵ�д�
 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�
�����Ŀ