��Ŀ����
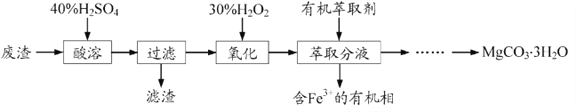
����Ŀ����Ʒ�Һ���ۺ����������ڼ����ؽ�����ˮ�����Ⱦ��ijʵ��С������������ģ������һ������ҵ��Ʒ�Һ(ǿ���ԣ���Cr2O72-������Cu2+��)�Ʊ�ˮ����Ƥ������Cr(OH)SO4���ش��������⣺
(��֪lg2=0.3��Ksp[ Cr(OH)3]=6.4��10-31, Ksp[ Cu(OH)2]=2��10-20 )
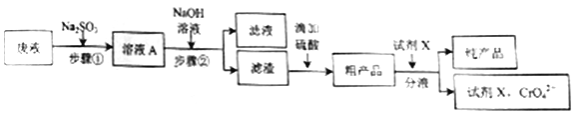
(1)��Na2SO3ǰ�Ƚ���Һ��Ϊ��������Һ��ԭ����__________________��д����Һ�м�Na2SO3ʱ������Ӧ�����ӷ���ʽ_____________��
(2)������뱣���¶���30��50�棬�ɲ��õĴ�ʩ��___________________��
(3)����ڵIJ���������___________��
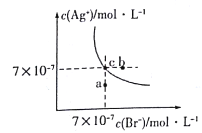
(4)���ڲ�����У�ʹCr3+��ȫ����������Һ��pH��ΧΪ______________,
[��֪��Һ��c(Cu2+)=2��10-4mol��L-1,��ȫ����ʱc(Cr3+)<10-5 mol��L-1]
(5)�ֲ�Ʒ�к�����CrO42-����ҵѡ����Լ�XӦ�����������__________��
A.��ˮ�������� B.CrO42-��X���ܽ��С
C.Cr(OH)SO4��X���ܽ�ȴ� D.Cr(OH)SO4��X����Ӧ
(6)����ҵ��Ʒ�Һ��0.1mol Cr2O72-�������Ƶò�Ʒ31.35g�������Ϊ__________��
(7)���������£����ǻ�ԭNa2Cr2O7Ҳ���Ʊ�Cr(OH)SO4��
Na2Cr2O7+NaHSO4+C12H22O11��Cr(OH)SO4+Na2SO4+H2O+CO2(δ��ƽ)
�ٷ�Ӧ��ÿ����1molCr(OH)SO4����������ҪC12H22O11_________mol
�ڽ�����Һ������17�����£����ã����ˣ���80��ʱ������Һ���õ����Ĺ�ҵ��Ʒ���ò�Ʒ�л��е���Ҫ������________________��
���𰸡� ��Һ���Թ�ǿ��Na2SO3����SO2�������ʽ��� Cr2O72-+3SO32-+8H+=2Cr3++3SO42-+4H2O ˮԡ���� ���� ϴ�� 5.6<pH<6 AD 95% 1/16 Na2SO4
��������(1)������Һ���Թ�ǿ��Na2SO3����SO2�������ʽ��ͣ����Լ�Na2SO3ǰ�Ƚ���Һ��Ϊ��������Һ���������ƾ��л�ԭ�ԣ��ܰ��ظ�����������������Һ�м�Na2SO3ʱ������Ӧ�����ӷ���ʽΪCr2O72-+3SO32-+8H+=2Cr3++3SO42-+4H2O��(2)������뱣���¶���30��50�棬�ɲ��õĴ�ʩ��ˮԡ���ȡ�(3)����ڵõ���������Һ������������ǹ��ˡ�(4)��ȫ����ʱc(Cr3+)<10-5 mol��L-1����ʱ��Һ��������Ũ����![]() ����Һ��������Ũ����2.5��10��6mol/L������pH��5.6����Һ��c(Cu2+)=2��10-4mol��L-1����ʼ������������Ũ����
����Һ��������Ũ����2.5��10��6mol/L������pH��5.6����Һ��c(Cu2+)=2��10-4mol��L-1����ʼ������������Ũ����![]() ��pH��6��������Һ��pH��ΧΪ5.6<pH<6��(5)Ҫ��ȥ�ֲ�Ʒ�к�����CrO42-�����Լ�XӦ�������������ˮ����������Cr(OH)SO4��X����Ӧ����ѡAD��(6)����ҵ��Ʒ�Һ��0.1mol Cr2O72-�����������Ƶ�Cr(OH)SO4��������0.2mol��165g/mol��33g�������Ƶò�Ʒ31.35g�������Ϊ31.35g/33g��100%��95.0%��(7)�ٷ�Ӧ��CrԪ�ػ��ϼ۴�+7�۽��͵�+3�ۣ��õ�3�����ӡ�̼Ԫ�ػ��ϼ۴�0�����ߵ�+4�ۣ����Ը��ݵ��ӵ�ʧ�غ��֪ÿ����1molCr(OH)SO4����������ҪC12H22O11�����ʵ�����3mol��48��1/16mol���ڷ�Ӧ�л������������ɣ���˸ò�Ʒ�л��е���Ҫ������Na2SO4��
��pH��6��������Һ��pH��ΧΪ5.6<pH<6��(5)Ҫ��ȥ�ֲ�Ʒ�к�����CrO42-�����Լ�XӦ�������������ˮ����������Cr(OH)SO4��X����Ӧ����ѡAD��(6)����ҵ��Ʒ�Һ��0.1mol Cr2O72-�����������Ƶ�Cr(OH)SO4��������0.2mol��165g/mol��33g�������Ƶò�Ʒ31.35g�������Ϊ31.35g/33g��100%��95.0%��(7)�ٷ�Ӧ��CrԪ�ػ��ϼ۴�+7�۽��͵�+3�ۣ��õ�3�����ӡ�̼Ԫ�ػ��ϼ۴�0�����ߵ�+4�ۣ����Ը��ݵ��ӵ�ʧ�غ��֪ÿ����1molCr(OH)SO4����������ҪC12H22O11�����ʵ�����3mol��48��1/16mol���ڷ�Ӧ�л������������ɣ���˸ò�Ʒ�л��е���Ҫ������Na2SO4��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�