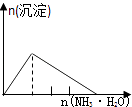��Ŀ����
����Ŀ����ʹ������к͵ζ����ⶨ���۰״�(��Ҫ�ɷ���CH3COOH)��������(g��100 mL��1)����֪CH3COOH + NaOH=====CH3COONa + H2O �յ�ʱ������Һ�ʼ��ԡ�
��.ʵ�鲽�裺
��1����________________(����������)��ȡ10.00 mLʳ�ð״�����__________(����������)����ˮϡ�ͺ�ת�Ƶ�100 mL__________(����������)�ж�����ҡ�ȼ��ô���״���Һ��

��2����_____ȡ����״���Һ20.00 mL����ƿ���������еμ�2��_____��ָʾ����
��3����ȡʢװ0.100 0 mol��L��1NaOH ��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ������ͼ��ʾ�����ʱ�Ķ���Ϊ________mL��
��4���ζ�����____________________ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Ρ�
��.ʵ���¼
ʵ�����ݣ�mL�� �ζ����� | 1 | 2 | 3 | 4 |
V(��Ʒ) | 20.00 | 20.00 | 20.00 | 20.00 |
V(NaOH)(����) | 15.95 | 15.00 | 15.05 | 14.95 |
��.���ݴ��������ۣ�
��1����ͬѧ�ڴ�������ʱ����ã�ƽ�����ĵ�NaOH��Һ�����V��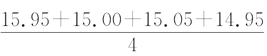 mL��15.24 mL��ָ��������IJ�����֮���� _______������ȷ���ݴ������ɵ�c(���۰״�)��________mol��L��1�����۰״���������________g��100 mL��1��
mL��15.24 mL��ָ��������IJ�����֮���� _______������ȷ���ݴ������ɵ�c(���۰״�)��________mol��L��1�����۰״���������________g��100 mL��1��
��2���ڱ�ʵ��ĵζ������������в�����ʹʵ����ƫ�����_________(��д���)��
A����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
B����ʽ�ζ��ܵļ����ڵζ�ǰ���������ζ���������ʧ
C����ƿ�м������״���Һ�����ټ�����ˮ
D����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
���𰸡� ��ʽ�ζ���(��10 mL��Һ��) �ձ� ����ƿ ��ʽ�ζ��� ��̪ 0.60 ��Һ����ɫǡ�ñ�Ϊdz��ɫ�����ڰ�����ڲ���ɫ ��1�εζ�������Դ����쳣ֵ��Ӧ��ȥ 0.75 4.50 ab
����������.��1���״�Ϊ������Һ��Ӧ��ʹ����ʽ�ζ�����ȡ�״���Һ�����ձ�����ˮϡ�ͺ�ת�Ƶ�100 mL����ƿ�С�
��2���״�Ϊ������Һ��Ӧ��ʹ����ʽ�ζ�����ȡ�״���Һ��ʳ����NaOH��Ӧ������ǿ�������Σ���Һ�ʼ��ԣ�Ӧѡ����Ա�ɫ��Χ�ڵ�ָʾ��������̪��
��3�������밼Һ����͵����ж���Ϊ0.60mL
��4������Һ����ɫǡ�ñ�Ϊdz��ɫ�����ڰ�����ڲ���ɫʱ��ֹͣ�ζ���
III����1����1�εζ�������Դ����쳣ֵ��Ӧ��ȥ��3�����ĵ�NaOH��Һ�����Ϊ��15.00mL��15.05mL��14.95mL����NaOH��Һ�������ƽ��ֵΪ����15.00mL+15.05mL+14.95mL��/3=15.00mL����10mL���۰״���Ʒ���� CH3COOOH xg����
CH3COOOH��NaOH
60 40
xg��0.2 0.1000mol/L��0.015L��40g/mol x=0.45
C(���۰״�)=0.45g/60g/mol/0.01L==0.75mol/L����Ʒ������Ϊ��4.50g/100mL��
�ʴ�Ϊ����1�εζ�������Դ����쳣ֵ��Ӧ��ȥ��0.75��4.50��
��2��Ũ��������������c�����⣩= c(��)V(��)/ V(����)���з�����
A����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ����ҺŨ�Ƚ��ͣ����V������ƫ����c�����⣩= c(��)V(��)/ V(����) ��֪��c�����⣩ƫ��A��ȷ��
B����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ����Һ�������c�����⣩ƫ��B��ȷ��
C����ƿ�м������״���Һ���ټ�����ˮ��������ʵ������䣬��Ӱ�죬��C����
D����ƿ�ڵζ�ʱ����ҡ����������Һ�彦�������ʼ��٣�Ũ�Ƚ��ͣ���D����
ƫ�ͣ���D����ѡAB��

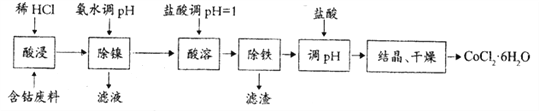
����Ŀ���ú��ܷ���(��CoCO3������NiCO3����м)�Ʊ�CoCl2��6H2O�Ĺ����������£�
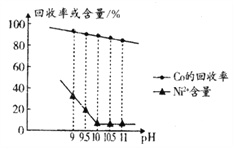
��֪��������������ҺpH��Co�Ļ�����Ӱ������ͼ��ʾ�����ֽ���������ʵ�������¿�ʼ��������ȫ������pH���±���ʾ��
�������� | ��ʼ������pH | ��ȫ������pH |
Fe3+ | 1.5 | 4.1 |
Fe2+ | 7.5 | 9.7 |
Co2+ | 6.6 | 9.4 |
��1���������������ϡHCl������˴�ͳ��������������Ļ����������ŵ�Ϊ__________________��
��2������ʱӦ����pH=______________���˲�����Ni2+�Ƿ��γ�Ni(OH)2������___________________ ��
��3���������̵IJ��裺_______________�����˵�CoCl2��Һ��(�ɹ�ѡ�õ��Լ���30% H2O2��1.0mol��L-1KMnO4��NaOH���塢CoCO3����)
��4����������������pH��������____________________��
��5����ҵ�ϲ��ü�ѹ���ɵķ����Ʊ�CoCl2��6H2O����ѹ���ɵ�Ŀ����___________________��
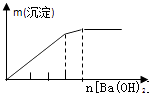
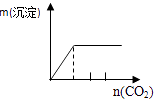
����Ŀ����ʵ�����Ӧʾ��ͼ�Ĺ�ϵ��ȷ���ǣ� ��
A | B | C | D |
NaAlO2��Һ����εμ����������� | AlCl3��Һ����εμӰ�ˮ������ | ����[KAl��SO4��212H2O]��Һ����εμ�Ba��OH��2��Һ������ | ����ʯ��ˮ�л���ͨ��CO2������ |
|
|
|
|
A.A
B.B
C.C
D.D