ƒøƒ⁄»ð
úÀ·∂˛º◊ı•(DMC) «“ª÷÷Ω¸ƒÍ¿¥ еΩπ„∑∫πÿ◊¢µƒª∑±£–լÅ´ªØπ§≤˙∆∑°£‘⁄¥þªØº¡◊˜”√œ¬£¨ø…”…º◊¥º∫ÕCO2÷±Ω”∫œ≥…DMC£∫CO2 + 2CH3OH °˙ CO(OCH3)2 + H2O£¨µ´º◊¥º◊™ªØ¬ Õ®≥£≤ªª·≥¨π˝1% «÷∆‘º∏√∑¥”¶◊þœÚ𧓵ªØµƒ÷˜“™‘≠“Ú°£ƒ≥—–æø–°◊È‘⁄∆‰À˚Ãıº˛≤ª±‰µƒ«Èøˆœ¬£¨Õ®π˝—–æøŒ¬∂»°¢∑¥”¶ ±º‰°¢¥þªØº¡”√¡ø∑÷±∂‘◊™ªØ ˝(TON)µƒ”∞œÏ¿¥∆¿º€¥þªØº¡µƒ¥þªØ–ßπ˚°£º∆À„π´ ΩŒ™£∫TON=◊™ªØµƒº◊¥ºµƒŒÔ÷ µƒ¡ø/¥þªØº¡µƒŒÔ÷ µƒ¡ø°£
£®1£©“—÷™25°Ê ±£¨º◊¥º∫ÕDMCµƒ±Í◊º»º…’»»∑÷±Œ™°˜H1∫Õ°˜H2£¨‘Ú…œ ˆ∑¥”¶‘⁄25°Ê ±µƒÏ ±‰°˜H3=_____°£
£®2£©∏˘æð∑¥”¶Œ¬∂»∂‘TONµƒ”∞œÏÕº£®œ¬◊ÛÕº£©≈–∂œ∏√∑¥”¶µƒÏ ±‰°˜H________0£®ÃÓ°∞>°±°¢°∞=°±ªÚ°∞<°±£©£¨¿Ì”… «________________________________°£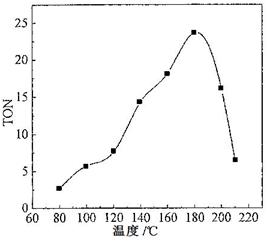
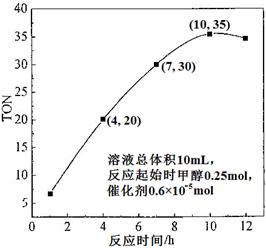
£®3£©∏˘æð∑¥”¶ ±º‰∂‘TONµƒ”∞œÏÕº£®…œ”“Õº£©£¨“—÷™»Ð“∫◊Ðê˝10mL£¨∑¥”¶∆ º ±º◊¥º0.25mol£¨¥þªØº¡0.6°¡10°™5 mol£¨º∆À„∏√Œ¬∂»œ¬£¨4°´7 hƒ⁄DMCµƒ∆Ωæ˘∑¥”¶ÀŸ¬ £∫________£ªº∆À„10 h ±£¨º◊¥ºµƒ◊™ªØ¬ £∫________°£
£®4£©∏˘æð∏√—–æø–°◊ȵƒ µ—Ⱥ∞¥þªØº¡”√¡ø∂‘TONµƒ”∞œÏÕº£®œ¬Õº£©£¨≈–∂œœ¬¡–Àµ∑®’˝»∑µƒ «___ __°£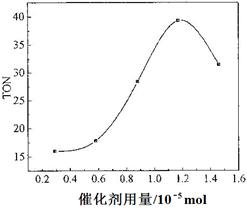
a. ”…º◊¥º∫ÕCO2÷±Ω”∫œ≥…DMC£¨ø…“‘¿˚”√º€¡Æ“◊µ√µƒº◊¥º∞—”∞œÏª∑æ≥µƒŒ¬ “∆¯ÃÂCO2◊™ªØŒ™◊ ‘¥£¨‘⁄◊ ‘¥—≠ª∑¿˚”√∫Õª∑æ≥±£ª§∑Ω√Ê∂ºæþ”–÷ÿ“™“‚“Â
b. ‘⁄∑¥”¶ÃÂœµ÷–Ã̺”∫œ µƒÕ—Àƺ¡£¨Ω´Ã·∏þ∏√∑¥”¶µƒTON
c. µ±¥þªØº¡”√¡øµÕ”⁄1.2°¡10°™5 mol ±£¨ÀÊ◊≈¥þªØº¡”√¡øµƒ‘ˆº”£¨º◊¥ºµƒ∆Ω∫‚◊™ªØ¬ œ‘÷¯Ã·∏þ
d. µ±¥þªØº¡”√¡ø∏þ”⁄1.2°¡10°™5 mol ±£¨ÀÊ◊≈¥þªØº¡”√¡øµƒ‘ˆº”£¨DMCµƒ≤˙¬ ∑¥∂¯º±æÁœ¬Ωµ
£®15∑÷£©
£®1£©2°˜H1 °™ °˜H2 £®3∑÷£©
£®2£©< £®2∑÷£© æ≠π˝œýÕ¨µƒ∑¥”¶ ±º‰£¨Œ¬∂»ΩœµÕ ±£¨∑¥”¶Œ¥¥ÔµΩ∆Ω∫‚£ªŒ¬∂»Ωœ∏þ ±£¨∑¥”¶“—¥ÔµΩ∆Ω∫‚£¨ÀÊ◊≈Œ¬∂»…˝∏þ£¨TONºı–°£¨º¥∆Ω∫‚œÚ◊Û“∆∂Ø£¨Àµ√˜∏√∑¥”¶∑≈»»£®3∑÷£©
£®3£©1°¡10-3 mol°§L-1°§h-1£® 2∑÷£© 8.4°¡10-2 % £®2∑÷£©
£®4£©ab£®3∑÷£©
Ω‚Œˆ ‘Â∑÷Œˆ£∫
£®1£©∏˘æð∑¥”¶¢ŸCH3OH(l)+1.5O2(g)=CO2(g)+2H2O(l) ¶§H1£¨¢⁄CO(OCH3)2(l)+3O2(g)=3CO2(g)+3H2O(l) ¶§H1£¨∏˘æð∏«Àπ∂®¬…”–ƒø±Í∑¥”¶µ»”⁄¢Ÿ°¡2-¢⁄£¨π ”–¶§H3=2¶§H1-¶§H2
£®2£©∏˘æðÕºø…÷™£¨Œ¬∂»…˝∏þ£¨∆Ω∫‚ƒÊœÚ“∆∂Ø£¨π ’˝∑¥”¶∑≈»»¶§H<0£ª
£®3£©4h£¨TON=20£¨7h£¨TON=30£¨¶§TON=10£¨¶§º◊¥º=10°¡0.6°¡10-5mol=0.6°¡10-4mol£¨ÀŸ¬ Œ™0.6°¡10-4mol°¬0.01L°¬3h=2°¡10-3 mol°§L-1°§h-1£¨10h£¨◊™ªØµƒº◊¥ºŒ™35°¡0.6°¡10-5mol=2.1°¡10-4mol£¨◊™ªØ¬ Œ™2.1°¡10-4mol°¬0.25mol=8.4°¡10-2 %
£®4£©a°¢’˝»∑£¨b°¢ºı…ŸÀÆ£¨ π∆Ω∫‚’˝œÚ“∆∂Ø£¨’˝»∑£ªcd°¢TON∫Õ◊™ªØ¬ ≤ª «“ª—˘£¨¥ÌŒÛ£ª
øºµ„£∫øº≤Ȫؗß∑¥”¶ÀŸ¬ º∞ªØ—ß∆Ω∫‚µ»œýπÿ÷™ ∂°£…ʺ∞–¬–≈œ¢µƒªÒ»°∫ÕΩ‚æˆŒ ƒС¶°£

 ◊€∫œ◊‘≤‚œµ¡–¥∞∏
◊€∫œ◊‘≤‚œµ¡–¥∞∏𧓵…œø…¿˚”√COªÚCO2¿¥…˙≤˙»º¡œº◊¥º°£“—÷™º◊¥º÷∆±∏µƒ”–πÿªØ—ß∑¥”¶“‘º∞‘⁄≤ªÕ¨Œ¬∂»œ¬µƒªØ—ß∑¥”¶∆Ω∫‚≥£ ˝»Áœ¬±ÌÀ˘ æ£∫
| ªØ—ß∑¥”¶ | ∆Ω∫‚≥£ ˝ | Œ¬∂»°Ê | ||
| 500 | 700 | 800 | ||
¢Ÿ2H2(g)+CO(g) CH3OH(g) CH3OH(g) | K1 | 2.5 | 0.34 | 0.15 |
¢⁄H2(g)+CO2(g) H2O (g)+CO(g) H2O (g)+CO(g) | K2 | 1.0 | 1.70 | 2.52 |
¢€3H2(g)+CO2(g) CH3OH(g)+H2O (g) CH3OH(g)+H2O (g) | K3 | | | |
«Îªÿ¥œ¬¡–Œ £∫
£®1£©∑¥”¶¢⁄ « £®ÃÓ°∞Œ¸»»°±ªÚ°∞∑≈»»°±£©∑¥”¶°£
£®2£©æð∑¥”¶¢Ÿ”΢⁄ø…Õ∆µº≥ˆK1°¢K2”ÎK3÷ƺ‰µƒπÿœµ£¨‘ÚK3= £®”√K1°¢K2±Ì 棩°£
£®3£©500°Ê ±≤‚µ√∑¥”¶¢€‘⁄ƒ≥ ±øã¨H2(g)°¢CO2(g)°¢CH3OH(g)°¢H2O (g)µƒ≈®∂»£®mol°§L-1£©∑÷±Œ™0.8°¢0.1°¢0.3°¢0.15£¨‘Ú¥À ±V’˝ VƒÊ£®ÃÓ°∞ > °±°¢°∞=°±ªÚ°∞<°±£©°£
£®4£©∑¥”¶¢Ÿ∞¥’’œýÕ¨µƒŒÔ÷ µƒ¡øÕ∂¡œ£¨≤‚µ√CO‘⁄≤ªÕ¨Œ¬∂»œ¬µƒ∆Ω∫‚◊™ªØ¬ ”Ηπ«øµƒπÿœµ»Áœ¬ÕºÀ˘ æ°£œ¬¡–Àµ∑®’˝»∑µƒ «_____________£®ÃÓ–Ú∫≈£©°£

A£ÆŒ¬∂»£∫T1£æT2£æT3
B£Æ’˝∑¥”¶ÀŸ¬ £∫¶‘(a)£æ¶‘(c) , ¶‘(b)£æ¶‘(d)
C£Æ∆Ω∫‚≥£ ˝£∫K(a)£æK(c) , K(b)=K(d)
D£Æ∆Ωæ˘ƒ¶∂˚÷ ¡ø£∫M(a)£æM(c) , M(b)£æM(d)
ÀÆ√∫∆¯◊™ªØ∑¥”¶CO(g)+H2O(g)  CO2(g)+H2 (g)‘⁄“ª∂®Œ¬∂»œ¬¥ÔµΩªØ—ß∆Ω∫‚◊¥Ã¨°£
CO2(g)+H2 (g)‘⁄“ª∂®Œ¬∂»œ¬¥ÔµΩªØ—ß∆Ω∫‚◊¥Ã¨°£
ÕÍ≥…œ¬¡–ÃÓø’£∫
£®1£©–¥≥ˆ∏√∑¥”¶µƒ∆Ω∫‚≥£ ˝±Ì¥Ô ΩK=________________°£
£®2£©“ª∂®Œ¬∂»œ¬£¨‘⁄“ª∏ˆ»ðª˝≤ª±‰µƒ√б’»ð∆˜÷–∑¢…˙…œ ˆ∑¥”¶£¨œ¬¡–Àµ∑®÷–ƒÐ≈–∂œ∏√∑¥”¶¥ÔµΩªØ—ß∆Ω∫‚◊¥Ã¨µƒ « £®—°ÃÓ±ý∫≈£©°£
a£Æ»ð∆˜÷–µƒ—π«ø≤ª±‰ b£Æ1 mol H£≠Hº¸∂œ¡—µƒÕ¨ ±∂œ¡—2 molH£≠Oº¸
c£Æv’˝(CO) = vƒÊ(H2O) d£Æc(CO) = c(H2)
£®3£©Ω´≤ªÕ¨¡øµƒCO(g)∫ÕH2O(g)∑÷±Õ®»ÎµΩê˝Œ™2Lµƒ∫„»ð√б’»ð∆˜÷–Ω¯––∑¥”¶£¨µ√µΩ»Áœ¬ µ—È◊È1∫Õ µ—È◊È2µƒ ˝æð£∫
| µ—È◊È | Œ¬∂»/°Ê | ∆ º¡ø/mol | ∆Ω∫‚¡ø/mol | ¥ÔµΩ∆Ω∫‚À˘–Ë ±º‰/min | ||
| H2O | CO | H2 | CO | |||
| 1 | 650 | 2 | 4 | 1.6 | 2.4 | 3 |
| 2 | 650 | 1 | 2 | 0.8 | 1.2 | 5 |
| 3 | 950 | 1 | 2 | °™ | °™ | °™ |
¢Ÿ”… µ—È◊È1µƒ ˝æðø…÷™£¨∆Ω∫‚ ±COµƒ◊™ªØ¬ Œ™ %°£
¢⁄”… µ—È◊È1∫Õ2µƒ ˝æðø…∑÷Œˆ£¨—π«ø∂‘∏√ø…ƒÊ∑¥”¶µƒ”∞œÏ « °£
¢€”–¡À µ—È◊È1∫Õ2µƒ ˝æ𣨑Ÿ…˺∆ µ—È◊È3£¨∆‰ƒøµƒ « °£
[15∑÷]º◊ÕÈ◊‘»»÷ÿ’˚ «œ»Ω¯µƒ÷∆«‚∑Ω∑®£¨∞¸∫¨º◊ÕÈ—ıªØ∫Õ’Ù∆˚÷ÿ’˚°£œÚ∑¥”¶œµÕ≥Õ¨ ±Õ®»Îº◊ÕÈ°¢—ı∆¯∫ÕÀÆ’Ù∆¯£¨∑¢…˙µƒ÷˜“™ªØ—ß∑¥”¶”–£∫
| ∑¥”¶π˝≥à | ªØ—ß∑Ω≥Ã Ω | Ï ±‰°˜H (kJ/mol) | ªÓªØƒÐEa (kJ/mol) |
| º◊ÕÈ—ıªØ | CH4(g)£´2O2(g) CO2(g)£´2H2O(g) CO2(g)£´2H2O(g) | £≠802.6 | 125.6 |
CH4(g)£´O2(g) CO2(g)£´2H2(g) CO2(g)£´2H2(g) | £≠322.0 | 172.5 | |
| ’Ù∆˚÷ÿ’˚ | CH4(g)£´H2O(g) CO(g)£´3H2(g) CO(g)£´3H2(g) | 206.2 | 240.1 |
CH4(g)£´2H2O(g) CO2(g)£´4H2(g) CO2(g)£´4H2(g) | 165.0 | 243.9 |
£®1£©∑¥”¶CO(g)£´H2O(g)
 CO2(g)£´H2(g)µƒ°˜H= kJ/mol°£
CO2(g)£´H2(g)µƒ°˜H= kJ/mol°££®2£©‘⁄≥ı ºΩ◊∂Œ,º◊ÕÈ’Ù∆˚÷ÿ’˚µƒ∑¥”¶ÀŸ¬ º◊ÕÈ—ıªØµƒ∑¥”¶ÀŸ¬ £®ÃÓ¥Û”⁄°¢–°”⁄ªÚµ»”⁄£©°£
£®3£©∂‘”⁄∆¯œý∑¥”¶£¨”√ƒ≥◊È∑÷(B)µƒ∆Ω∫‚—π«ø(PB)¥˙ÃÊŒÔ÷ µƒ¡ø≈®∂»(cB)“≤ø…“‘∆Ω∫‚≥£ ˝£®º«◊˜KP£©£¨‘Ú∑¥”¶CH4(g)£´H2O(g)
 CO(g)£´3H2(g)µƒKP£Ω £ª
CO(g)£´3H2(g)µƒKP£Ω £ªÀÊ◊≈Œ¬∂»µƒ…˝∏þ£¨∏√∆Ω∫‚≥£ ˝ £®ÃÓ°∞‘ˆ¥Û°±°¢°∞ºı–°°±ªÚ°∞≤ª±‰°±£©°£
£®4£©¥”ƒÐ¡øΩ◊∂Œ∑÷Œˆ£¨º◊ÕÈ◊‘»»÷ÿ’˚∑Ω∑®µƒœ»Ω¯÷Æ¥¶‘⁄”⁄ °£
£®5£©‘⁄ƒ≥“ª∏¯∂®Ω¯¡œ±»µƒ«Èøˆœ¬£¨Œ¬∂»°¢—π«ø∂‘H2∫ÕCOŒÔ÷ µƒ¡ø∑÷ ˝µƒ”∞œÏ»Áœ¬Õº£∫
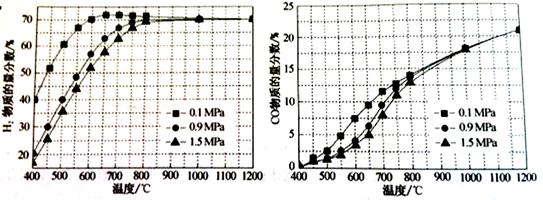
¢Ÿ»Ù“™¥ÔµΩH2ŒÔ÷ µƒ¡ø∑÷ ˝>65%°¢COµƒŒÔ÷ µƒ¡ø∑÷ ˝<10%£¨“‘œ¬Ãıº˛÷–◊Ó∫œ µƒ « °£
A£Æ600°Ê£¨0.9Mpa B£Æ700°Ê£¨0.9MPa C£Æ800°Ê£¨1.5Mpa D£Æ1000°Ê£¨1.5MPa
¢⁄ª≠≥ˆ600°Ê£¨0.1MpaÃıº˛œ¬£¨œµÕ≥÷–H2ŒÔ÷ µƒ¡ø∑÷ ˝ÀÊ∑¥”¶ ±º‰£®¥”≥£Œ¬Ω¯¡œø™ ºº¥ ±£©
µƒ±‰ªØ«˜ ∆ æ“‚Õº£∫
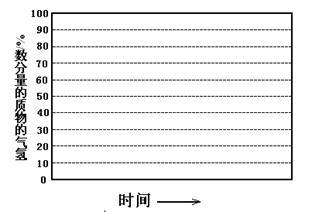
£®6£©»Áπ˚Ω¯¡œ÷–—ı∆¯¡øπ˝¥Û£¨◊Ó÷’µº÷¬H2ŒÔ÷ µƒ¡ø∑÷ ˝ΩµµÕ£¨‘≠“Ú « °£
 2SO3(g)£¨∆Ω∫‚ªÏ∫œÃÂœµ÷–SO3µƒ∞Ÿ∑÷∫¨¡ø∫ÕŒ¬∂»µƒπÿœµ»ÁÕº
2SO3(g)£¨∆Ω∫‚ªÏ∫œÃÂœµ÷–SO3µƒ∞Ÿ∑÷∫¨¡ø∫ÕŒ¬∂»µƒπÿœµ»ÁÕº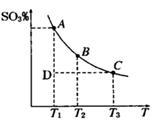 À˘ 棨∏˘æðÕºªÿ¥œ¬¡–Œ £∫
À˘ 棨∏˘æðÕºªÿ¥œ¬¡–Œ £∫

 2B(s) + 6HBr(g) ¿¥÷∆»°æßÃÂ≈°£ÕÍ≥…œ¬¡–ÃÓø’£∫
2B(s) + 6HBr(g) ¿¥÷∆»°æßÃÂ≈°£ÕÍ≥…œ¬¡–ÃÓø’£∫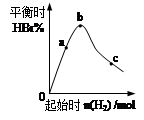
 N2O3(g) ¶§H<0
N2O3(g) ¶§H<0 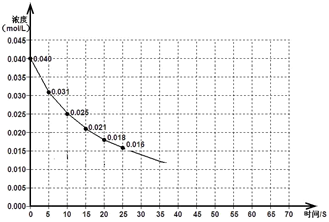
 C(g)£´D(g)¥ÔµΩ∆Ω∫‚∫Û£¨…˝∏þŒ¬∂»»ð∆˜ƒ⁄∆¯Ãµƒ√Ð∂»‘ˆ¥Û°£«Îªÿ¥œ¬¡–Œ £∫
C(g)£´D(g)¥ÔµΩ∆Ω∫‚∫Û£¨…˝∏þŒ¬∂»»ð∆˜ƒ⁄∆¯Ãµƒ√Ð∂»‘ˆ¥Û°£«Îªÿ¥œ¬¡–Œ £∫