��Ŀ����
20����1��8.8��CO2�����ʵ�����0.2mol������Oԭ�ӵ���ĿΪ0.2NA����״���µ������4.48L����2��19��MgCl2��Mg2+���ʵ�����0.2mol������������Ŀ0.6NA������Cl-0.4mol��MgCl2��Mg2+��������4.8g��
��3��3.01��1023��NH4+�����ʵ���Ϊ0.5mol������Ϊ9g���������ӵ����ʵ���Ϊ5.5mol�����е��ӵ���ĿΪ5NA��
��4��1.5mol SO3������Ϊ120g�����״����50.4L SO2������ͬ��Ŀ����ԭ�ӣ�16g ��������11.2L�������������ȼ�տ���ȫ��Ӧ��
��5����ͬ��������������Cl2��O2�����ǵ����ʵ�������32��71��������������������32��71������ͬ���������ǵ��������32��71��
��6����ͬ��ͬѹ�£�ͬ����ļ��飨CH4���Ͷ�����̼������֮��Ϊ1��1�����ʵ���֮��Ϊ1��1��ԭ������֮��Ϊ4��3������֮��Ϊ4��11��������ļ��飨CH4���Ͷ�����̼�Ļ�������ƽ��Ħ��������30g/mol��
���� ��1������n=$\frac{m}{M}$�����������̼�����ʵ������ٸ���n=nNA�����������ԭ����Ŀ������V=nVm���������¶�����̼�������
��2������n=$\frac{m}{M}$������Ȼ�þ�����ʵ������Ӷ��ó�þ���ӵ����ʵ���������n=nNA���������������Ŀ�������Ȼ�þ�Ļ�ѧʽ���������þ���ӵ����ʵ������ٸ���m=nM�����������
��3������n=$\frac{N}{{N}_{A}}$�����笠����ӵ����ʵ���������m=nM�����笠����ӵ�������笠������к���11�����ӡ�10�����ӣ����������ʵ���������������ʵ����ʵ�����n=nNA��������е�����Ŀ��
��4������m=nM�������������������������������������ʵ���������V=nVm���������¶���������������ȼ�����ɶ������������ԭ�ӵ����ʵ������ó��������������ʵ������ټ��������������������
��5����ͬ����ʱ�����ʵ�����Ħ�������ɷ��ȣ������������ʵ��������ȣ���ͬ���������֮�ȵ������ʵ���֮�ȣ�
��6����ͬ�����£�����������������ͬ�����ʵ����������������ݼ���Ͷ�����̼�ķ�����ɼ��������ԭ����֮�ȣ�����m=nM��������ߵ�����֮�ȣ�������ļ���Ͷ�����̼������ͬ�����ʵ���������M=$\frac{m}{n}$�����ƽ��Ħ��������
��� �⣺��1��8.8��CO2�����ʵ���Ϊ��$\frac{8.8g}{44g/mol}$=0.2mol��0.2mol������̼�����к���Oԭ�ӵ���ĿΪ��0.2NA����״����0.2mol������ǣ�22.4L/mol��0.2mol=4.48L��
�ʴ�Ϊ��0.2mol��0.2NA��4.48L��
��2��19��MgCl2�����ʵ���Ϊ��$\frac{19g}{95g/mol}$=0.2mol��0.2mol�Ȼ�þ�к���0.2molMg2+��0.2mol�Ȼ�þ�к���0.2molþ���ӡ�0.4mol�����ӣ��ܹ�����0.6mol���ӣ�����������Ŀ0.6NA������Cl-0.4mol��MgCl2��Mg2+�����ʵ���Ϊ��0.4mol��$\frac{1}{2}$=0.2mol��0.2molþ���ӵ�����Ϊ��24g/mol��0.2mol=4.8g��
�ʴ�Ϊ��0.2mol��0.6NA��4.8g��
��3��3.01��1023��NH4+�����ʵ���Ϊ��$\frac{3.01��1{0}^{23}}{6.02��1{0}^{23}}$=0.5mol������Ϊ��18g/mol��0.5mol=9g��0.5mol笠������к������ӵ����ʵ���Ϊ��0.5mol��11=5.5mol�����е��ӵ���ĿΪ��0.5��10��NA=5NA��
�ʴ�Ϊ��0.5mol��9g��5NA��
��4��1.5mol SO3������Ϊ��80g/mol��1.5mol=120g��1.5mol���������к�����ԭ�ӵ����ʵ���Ϊ��1.5mol��3=4.5mol������4.5mol��ԭ����Ҫ������������ʵ���Ϊ��$\frac{4.5mol}{2}$=2.25mol����״����2.25molSO2�����Ϊ��22.4L/mol��2.25mol=50.4L��16g����������ʵ���Ϊ��$\frac{16g}{32g/mol}$=0.5mol��0.5molS��ȫȼ������0.5mol����������Ҫ����0.5mol�����������0.5mol���������Ϊ��22.4L/mol��0.5mol=11.2L��
�ʴ�Ϊ��120g��50.4��11.2��
��5����ͬ��������������Cl2��O2�����ݸ���n=$\frac{m}{M}$��֪�����ǵ����ʵ�������Ħ�������ɷ��ȣ�����ߵ����ʵ���֮��Ϊ��32g/mol��71g/mol=32��71����������������=���ʵ���������=32��71������ͬ���������ǵ������=���ʵ���֮��=32��71��
�ʴ�Ϊ��32��71��32��71��32��71��
��6����ͬ��ͬѹ�£�ͬ����ļ��飨CH4���Ͷ�����̼������ͬ�����ʵ��������ߵķ�������ȣ��������֮��Ϊ1��1�����ʵ���֮��Ϊ1��1��ԭ������֮��Ϊ��1��4������1��3��=4��3�����ߵ����ʵ�����ȣ�������֮����Ħ�����������ȣ�����ߵ�����֮��Ϊ��16g/mol��44g/mol=4��11��������ļ��飨CH4���Ͷ�����̼������ͬ�����ʵ��������������ƽ��Ħ������Ϊ��$\frac{16g/mol+44g/mol}{1+1}$=30g/mol��
�ʴ�Ϊ��1��1��1��1��4��3��4��11��30g/mol��
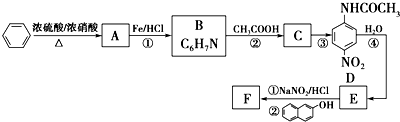
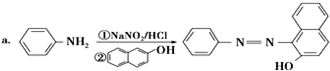
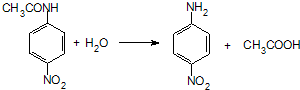
���� ���⿼���˰���٤�����ɼ������۵��ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ�ע�����հ���٤���������ݣ������������ʵ���������������֮���ת����ϵ������֪ʶ��϶ࡢ�������ϴ�ֿ���ѧ���ķ�����������ѧ����������

 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д�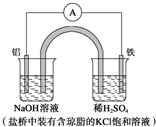
| A�� | Fe������������������Ӧ | |
| B�� | ������Ӧ��Al-3e-+3OH-�TAl��OH��3�� | |
| C�� | ����һ��ʱ���ʢ��ϡ������Һ�ı���pH���� | |
| D�� | �����е�Cl-������ձ����ƶ���ʹ���ձ�����Һ���ֵ����� |
�ٽ�ͭƬ���ڵ�ص������ϣ�
�ڽ���Ƭ���ڵ�Դ�������ϣ�
����ͭƬ�Ϸ����ķ�Ӧ�ǣ�Ag++e-�TAg��
������Ƭ�Ϸ����ķ�Ӧ�ǣ�4OH--4e-�TO2+2H2O��
������CuSO4��Һ��
������AgNO3��Һ�����Һ��
| A�� | �ڢۢ� | B�� | �٢ۢ� | C�� | �٢ܢ� | D�� | �ڢۢܢ� |
| A�� | C60������I2�����˷�����������ͬ | |
| B�� | �������������ķ���ʽ��ͬ�����ǵ��۵���� | |
| C�� | �Ȼ��ƺ��Ȼ�������ˮʱ���ƻ��Ļ�ѧ���������Ӽ� | |
| D�� | �������½ṹ�մɲ��ϵĹ����Ƿ��Ӿ��� |
 ��
��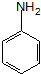 ����Ʒ�Ӧ�ںܵ͢�Ŀ���DZ�����������������
����Ʒ�Ӧ�ںܵ͢�Ŀ���DZ�����������������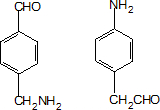 ��
��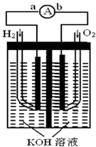 ����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ���õ�ص缫�����һ��ϸС�IJ��ۣ����������������ǿ�������ȶ�����ش�
����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ���õ�ص缫�����һ��ϸС�IJ��ۣ����������������ǿ�������ȶ�����ش� ��֪A��B��C��D��E����Ԫ�ص�ԭ������������������Aԭ����������������������������ԭ��������ȣ�Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��Dԭ��L���Ӳ�����2�ԳɶԵ��ӣ�E+ԭ�Ӻ�����3������Ҹ��������ȫ��״̬��
��֪A��B��C��D��E����Ԫ�ص�ԭ������������������Aԭ����������������������������ԭ��������ȣ�Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��Dԭ��L���Ӳ�����2�ԳɶԵ��ӣ�E+ԭ�Ӻ�����3������Ҹ��������ȫ��״̬�� ��
��