��Ŀ����
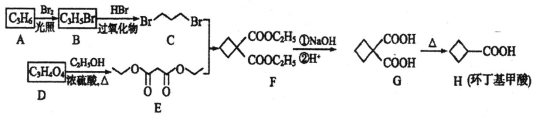
����Ŀ��A(C3H6)�ǻ����л�����ԭ�ϣ���A�Ʊ��ۺ���C�� �ϳ�·����ͼ��ʾ������������ȥ����
�ϳ�·����ͼ��ʾ������������ȥ����

��֪�� ��
��![]() R-COOH
R-COOH
(1)A��������_____________��B���������������________________��
(2)C�Ľṹ��ʽ________________��D��E�ķ�Ӧ����Ϊ________________
(3)E��F�Ļ�ѧ����ʽΪ___________________________��
(4)B��ͬ���칹���У���B������ͬ���������ܷ���������Ӧ�����к˴Ź�����������ʾ3��壬�ҷ����֮��Ϊ6:1:1����__________________��д���ṹ��ʽ����
(5)�����ʵ����� �ֱ�������NaOH��NaHCO3 ��Ӧ������NaOH��NaHCO3 �����ʵ���֮��Ϊ__________������
�ֱ�������NaOH��NaHCO3 ��Ӧ������NaOH��NaHCO3 �����ʵ���֮��Ϊ__________������ ����һ�ֹ����ŵķ�����______________��д�����������ơ���Ӧ�Լ�������
����һ�ֹ����ŵķ�����______________��д�����������ơ���Ӧ�Լ�������
���𰸡���ϩ ����  ȡ����Ӧ CH2=CH-CH2OH+CH2=CH-CCl=CH2
ȡ����Ӧ CH2=CH-CH2OH+CH2=CH-CCl=CH2![]()
![]() HCOO-CH=C(CH3)2 l��l �����Ȼ���ȡ�������л������������ɫʯ����Һ���(�����̼̼˫����������ˮ����ˮ��ɫ)
HCOO-CH=C(CH3)2 l��l �����Ȼ���ȡ�������л������������ɫʯ����Һ���(�����̼̼˫����������ˮ����ˮ��ɫ)
��������
A����ʽΪC3H6��A��CO��CH3OH������Ӧ����B����A�ṹ��ʽΪCH2=CHCH3��B�ṹ��ʽΪCH3CH=CHCOOCH3��B�����Ӿ۷�Ӧ���ɾ۶�ϩ��������۶�ϩ���������ˮ�ⷴӦ��Ȼ���ữ�õ��ۺ���C��C�ṹ��ʽΪ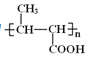 ��A��Cl2�ڸ����·�����Ӧ����D��D����ˮ�ⷴӦ����E������E�Ľṹ��ʽCH2=CHCH2OH��֪D�ṹ��ʽΪCH2=CHCH2Cl��E��2-��-1��3-����ϩ�����ӳɷ�Ӧ����F��F�ṹ��ʽΪ
��A��Cl2�ڸ����·�����Ӧ����D��D����ˮ�ⷴӦ����E������E�Ľṹ��ʽCH2=CHCH2OH��֪D�ṹ��ʽΪCH2=CHCH2Cl��E��2-��-1��3-����ϩ�����ӳɷ�Ӧ����F��F�ṹ��ʽΪ![]() ��F����ȡ����Ӧ����G��G������Ϣ�з�Ӧ�õ�����G�ṹ��ʽΪ
��F����ȡ����Ӧ����G��G������Ϣ�з�Ӧ�õ�����G�ṹ��ʽΪ![]() ��
��
���������ƶϿ�֪A��CH2=CH-CH3��CΪ ��D��CH2=CHCH2Cl��EΪCH2=CHCH2OH��F��
��D��CH2=CHCH2Cl��EΪCH2=CHCH2OH��F��![]() ��G��
��G��![]() ��
��
(1)A��CH2=CH-CH3������Ϊ��ϩ��B�ṹ��ʽΪCH3CH=CHCOOCH3��B�к���������������������
(2)C�Ľṹ��ʽΪ ��D��CH2=CHCH2Cl������Clԭ�ӣ���NaOH��ˮ��Һ���ȷ���ˮ�ⷴӦ����E��CH2=CHCH2OH����ˮ�ⷴӦҲ��ȡ����Ӧ�����D��ΪE�ķ�ӦΪȡ����Ӧ��ˮ�ⷴӦ��
��D��CH2=CHCH2Cl������Clԭ�ӣ���NaOH��ˮ��Һ���ȷ���ˮ�ⷴӦ����E��CH2=CHCH2OH����ˮ�ⷴӦҲ��ȡ����Ӧ�����D��ΪE�ķ�ӦΪȡ����Ӧ��ˮ�ⷴӦ��
(3)EΪCH2=CHCH2OH��E��2-��-1��3-����ϩ�����ӳɷ�Ӧ����F��F�ṹ��ʽΪ![]() ���÷�Ӧ����ʽΪ��CH2=CH-CH2OH+CH2=CH-CCl=CH2
���÷�Ӧ����ʽΪ��CH2=CH-CH2OH+CH2=CH-CCl=CH2![]()
![]() ��
��
(4)B�ṹ��ʽΪCH3CH=CHCOOCH3��B��ͬ���칹���У���B������ͬ���������ܷ���������Ӧ��˵������ȩ����������̼̼˫������Ϊ�����γɵ��������к˴Ź�����������ʾ3��壬�ҷ����֮��Ϊ6:1:1����HCOO-CH=C(CH3)2��
(5) �����Ȼ���������NaOH��Ӧ����
�����Ȼ���������NaOH��Ӧ���� �������Ȼ�������NaHCO3��Ӧ����
�������Ȼ�������NaHCO3��Ӧ���� ��H2O��CO2��������ʵ���
��H2O��CO2��������ʵ��� ����NaOH��NaHCO3�����ʵ���֮��Ϊ1��1����
����NaOH��NaHCO3�����ʵ���֮��Ϊ1��1���� �к����Ȼ���̼̼˫�������ǻ����ֹ����ţ������Ȼ��ķ����ǣ�ȡ�������л������������ɫʯ����Һ��Ϊ��ɫ������̼̼˫���ķ����ǣ�������ˮ����ˮ��ɫ��
�к����Ȼ���̼̼˫�������ǻ����ֹ����ţ������Ȼ��ķ����ǣ�ȡ�������л������������ɫʯ����Һ��Ϊ��ɫ������̼̼˫���ķ����ǣ�������ˮ����ˮ��ɫ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�