��Ŀ����
����Ŀ����֪t ��ʱ��0.01 mol/L NaOH��Һ��pH��11��0.1 mol/L��HA��Һ��c(H+)/c(OH-)=109
��ش��������⣺
��1�����¶��£�ˮ�����ӻ�Kw��_____��0.1 mol/L��HA��Һ��ˮ�������c(OH-)=_____��
��2���������£���pH֮��Ϊ14��NaOH��Һ��HA��Һ�������Ϻ�������Һ��_____(��ᡱ������С�)�ԡ�
��3���������£�����ˮϡ��0.01 mol/L HA��Һʱ�����гʼ�С���Ƶ���_____��
A��ˮ�ĵ���̶� B��c(HA)/c(A-)
C����Һ��c(H��)��c(OH��)�ij˻� D����Һ��c(A��)��c(HA)��ֵ
��4�������£�ȡpH��2�������HA��Һ��100 mL�������зֱ����������Zn������Ӧ����������Һ��pH�仯��ͼ��ʾ��
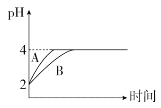
��ͼ�б�ʾHA��ҺpH�仯���ߵ���____(�A����B��)��
���������м���Zn������Ϊm1��HA��Һ�м���Zn������Ϊm2����m1_____m2(�>����<������)��
��5�������£�ȡ0.01 mol/L�������HA��Һ��100 mL���ֱ�μ�0.01 mol/LNaOH��Һ��ǡ����ȫ��Ӧ������NaOH��Һ�����ǰ��____����(�>����<������)��
���𰸡�1��10��13�� 10��11mol/L �� BD B < ��
��������
��1��0.01mol��L-1NaOH��Һ��c��OH����=10-2mol��L��1����֪pH=11������Kw�Ĺ�ʽ���㣻
0.1mol��L-1��HA��Һ��c(H��)/c(OH��)=109������Kw����c��H�������ټ���ˮ����ĵ�c(OH-)��
��2���������£�pH֮��Ϊ14��NaOH��Һ��HA��Һ��c��OH����=c��H�����������������ᣬ���Ũ�ȴ���������Ũ�ȣ�
��3��HA��������ʣ���ˮϡ��HA���ٽ�HA���룬��n��A������n��H��������n��HA����С����������ӡ�������Ũ������ij̶�С����Һ�������ij̶ȣ�����c��A������c��H������c��HA������С��
��4��HA��������ʣ��ڷ�Ӧ������HA��������������ӣ���Ӧ������HA��pH����̶�С��HCl������ȫ��Ӧ��HA��������pH��ȣ���HA��Ӧ�����ʵ����������ᣬ�������п֮��Ĺ�ϵ���㣮
��5��0.01 mol��L��1�������HA��Һ��100 mL�����ṩ����������ͬ�����ĵļ���ͬ��
��1��0.01mol��L-1NaOH��Һ��c��OH����=10-2mol��L��1����֪pH=11����c��H����=10-11mol��L��1����Kw=c��OH������c��H����=10-13��
0.1mol��L-1��HA��Һ��c(H��)/c(OH��)=109����֪Kw=10-13����c��H����=10-2mol��L��1��c(OH-)=10-11mol��L��1��
��2���������£�pH֮��Ϊ14��NaOH��Һ��HA��Һ������������Һ��c��OH�������ڴ�����Һ��c��H�����������������ᣬ���Ũ�ȴ���������Ũ�ȣ����Ի�Ϻ�����ʣ�࣬����Һ�����ԣ�
��3��A����ˮϡ�ͣ���Һ���������c��H������С����ˮ�ĵ�������Ƴ̶ȼ�С������ˮ�ĵ���̶�����A����
B����ˮϡ�ͣ��ٽ����룬��n��A��������n��HA����С������c(HA)/c(A��)��С����B��ȷ��
C����Һ��c��H������c��OH�����ij˻�Ϊ�������¶Ȳ��䣬�� c��H������c��OH�����ij˻����䣬��C����
D����ˮϡ�ͣ���Һ���������c��A������c��HA������С��������Һ��c��A������c��HA����ֵ��С����D��ȷ��
��ѡBD��
��4������HA��������ʣ��ڷ�Ӧ������HA����������ӣ����Է�Ӧ������HA��C��H��������HCl����HA��pH����̶�С��HCl�����Ա�ʾHA��Һ��pH�仯����ΪB��
�ڵ���ȫ��Ӧ��HA��������pH��ȣ���μӷ�Ӧ��n��HA����n��HCl���������ĵ�Խ�࣬��μӷ�Ӧ��Zn������Խ������m1��m2��
��5��0.01 mol��L��1�������HA��Һ��100 mL�����ṩ����������ͬ�����ĵļ���ͬ�������£�ȡ0.01 mol/L�������HA��Һ��100 mL���ֱ�μ�0.01 mol/LNaOH��Һ��ǡ����ȫ��Ӧ������NaOH��Һ�����ǰ�ߵ��ں��ߡ�

����Ŀ����1����������4�����ʣ���NO����Cl2����ŨH2SO4����NH4HCO3�����У������ֽ����______________��������ţ���ͬ���������������������______________���������������______________��
��2��ѡ��װ�������ʵ����
|
|
|
A | B | C |
����CCl4��ȡ��ˮ�е�I2��ѡ��_________________������ţ���ͬ����
������100 mL 0.50 mol/L NaOH��Һ��ѡ��_________________��
����NaCl��Һ�л�ȡNaCl���壬ѡ��____________________��
����Ŀ����һ���¶��£�������X������Y��2 mol����ij10 L�����ܱ������У�������ӦX(g)+Y(g) ![]() 2Z(g) ��H<0������ʱ���ﵽƽ�⡣��Ӧ�����вⶨ���������±���
2Z(g) ��H<0������ʱ���ﵽƽ�⡣��Ӧ�����вⶨ���������±���
t/min | 2 | 4 | 10 | 12 |
n(Y)/mol | 1.40 | 1.10 | 0.40 | 0.40 |
����˵����ȷ����
A. ��Ӧǰ2 min ��ƽ������v(Z)=3.0xl0-2 mol L-1min-1
B. ���¶��´˷�Ӧ��ƽ�ⳣ��K=64
C. ƽ��������������䣬�ٳ���2 mol Z����ƽ��ʱX�������������
D. ��Ӧ���е�10 minʱ���������������䣬�����¶ȣ���Ӧ�ﵽ��ƽ��ǰv(��)>v(����







