��Ŀ����
��ҵ�ϳɰ��ķ�ӦN2��3H2�� 2NH3�������仯����ͼ��ʾ����ش��й����⣺
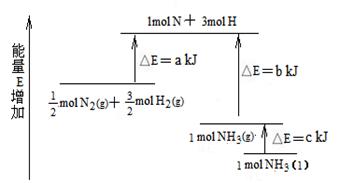
��1�����ϳ� 1 mol NH3(l) ____________������ա������ų�����_____________kJ��������
��2������֪���� lmol H��H ����lmol N��H ����lmol N��N ���ֱ���Ҫ��������436kJ��391kJ��946kJ������ͼ�еģ_______________kJ��1 mol N2(g) ��ȫ��Ӧ����NH3(g)�����������仯Ϊ ______KJ��
��3�����ƲⷴӦ 2NH3(l)�� N2(g)��3H2(g) �ȷ�Ӧ 2NH3 (g)�� N2(g)��3H2(g)______________������ա������ų�����������_____________����ࡱ�����١�����

��1�����ϳ� 1 mol NH3(l) ____________������ա������ų�����_____________kJ��������
��2������֪���� lmol H��H ����lmol N��H ����lmol N��N ���ֱ���Ҫ��������436kJ��391kJ��946kJ������ͼ�еģ_______________kJ��1 mol N2(g) ��ȫ��Ӧ����NH3(g)�����������仯Ϊ ______KJ��
��3�����ƲⷴӦ 2NH3(l)�� N2(g)��3H2(g) �ȷ�Ӧ 2NH3 (g)�� N2(g)��3H2(g)______________������ա������ų�����������_____________����ࡱ�����١�����
��1���ų� b+c-a
��2��1127 92
��3������ ��
��2��1127 92
��3������ ��
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
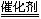 (3��2x)N2��6xH2O
(3��2x)N2��6xH2O (g) ��H��-92.4 kJ��mol-1
(g) ��H��-92.4 kJ��mol-1

 ����
���� ��1����֪��
��1����֪�� H1=" 1175.7" kJ��mol-1
H1=" 1175.7" kJ��mol-1
 H3="482.2" kJ��mol-1
H3="482.2" kJ��mol-1  J���÷�Ӧ���Ȼ�ѧ����ʽΪ________��
J���÷�Ӧ���Ȼ�ѧ����ʽΪ________�� O2��g��=H2O��1�� ��H=-285.8kJ��mol-1
O2��g��=H2O��1�� ��H=-285.8kJ��mol-1