��Ŀ����
��3�֣���úת��Ϊˮú����CO��H2�Ļ�����壩��ͨ����ѧ������úת��Ϊ�ྻȼ�ϵķ���֮һ��úת��Ϊˮú������Ҫ��ѧ��ӦΪ��C(s) + H2O(g)��CO(g) + H2(g)����H1�� ��֪��
��2H2(g) + O2(g) �� 2H2O(g)����H2����483.6kJ��mol��1
��2C(s) + O2(g) �� 2 CO(g)����H3����221.0kJ��mol��1
��������Ȼ�ѧ����ʽ������ó���H1�� ��
��2H2(g) + O2(g) �� 2H2O(g)����H2����483.6kJ��mol��1
��2C(s) + O2(g) �� 2 CO(g)����H3����221.0kJ��mol��1
��������Ȼ�ѧ����ʽ������ó���H1�� ��
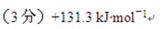
��

��ϰ��ϵ�д�
�����Ŀ
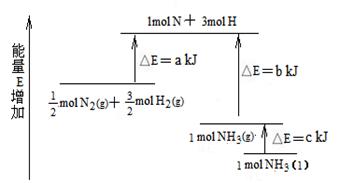
 3H2(g)��N2(g) ��H="(3a+c-6b)" kJ/mol
3H2(g)��N2(g) ��H="(3a+c-6b)" kJ/mol 
 4������a mol CH4��CO��H2���������ȫȼ�գ�����CO2�����Һ̬ˮ����CO2��H2O��
4������a mol CH4��CO��H2���������ȫȼ�գ�����CO2�����Һ̬ˮ����CO2��H2O��