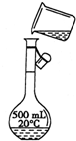
��Ŀ����
ʵ������Ҫ250mL1mol?L-1��Na2CO3��Һ����������������
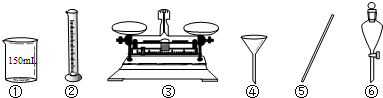
��1����Na2CO3����������Һʱ�����������б���ʹ�õ�����______������ţ���
��2����Ҫʵʩ���ƣ������������⣬��ȱ����������Ʒ��______��______�ȣ����������ƣ���
��3��������Һʱ��һ����Է�Ϊ���¼����������裺
A��ת��B������C��ϴ��D������E���ܽ�F��ҡ��G����ȴ
����ȷ�IJ���˳��Ӧ��BE______DF��������ţ���
��4�����ƹ����У���������������ȷ�����в���������Ũ��ƫ�����______��
A������ҡ�Ⱥ���Һ����ڿ̶���B������ʱ��������ƿ�Ŀ̶���
C������Һת������ƿ��û��ϴ���ձ��Ͳ���������ת�붨�ݲ���
D�������ˮ�����˿̶��ߣ�ȡ������ˮʹҺ��ǡ�õ��̶���
��5���ɼ����֪���ڳ��������У�����������ƽ����Na2CO3���������Ϊ______������5mol?L-1Na2CO3��Һ���Ƹ���Һ����Ӧ����Ͳ��ȡ����Һ�����Ϊ______��

��1����Na2CO3����������Һʱ�����������б���ʹ�õ�����______������ţ���
��2����Ҫʵʩ���ƣ������������⣬��ȱ����������Ʒ��______��______�ȣ����������ƣ���
��3��������Һʱ��һ����Է�Ϊ���¼����������裺
A��ת��B������C��ϴ��D������E���ܽ�F��ҡ��G����ȴ
����ȷ�IJ���˳��Ӧ��BE______DF��������ţ���
��4�����ƹ����У���������������ȷ�����в���������Ũ��ƫ�����______��
A������ҡ�Ⱥ���Һ����ڿ̶���B������ʱ��������ƿ�Ŀ̶���
C������Һת������ƿ��û��ϴ���ձ��Ͳ���������ת�붨�ݲ���
D�������ˮ�����˿̶��ߣ�ȡ������ˮʹҺ��ǡ�õ��̶���
��5���ɼ����֪���ڳ��������У�����������ƽ����Na2CO3���������Ϊ______������5mol?L-1Na2CO3��Һ���Ƹ���Һ����Ӧ����Ͳ��ȡ����Һ�����Ϊ______��
��1�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�����������������ƽ���ձ�����������250mL����ƿ����ͷ�ιܡ�ҩ�ף������ṩ���������������б���ʹ�õ����Т٢ۢݣ�
�ʴ�Ϊ���٢ۢݣ�
��2��ʵ������������������ƽ���ձ�����������250mL����ƿ����ͷ�ιܡ�ҩ�ף������������⣬��ȱ����������Ʒ��250mL����ƿ ��ͷ�ιܣ�
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��3�����������У����㡢�������ܽ⡢��ȴ����Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ȷ�IJ���˳��Ӧ��BEGACADF��
�ʴ�Ϊ��GACA��
��4��A������ҡ�Ⱥ���Һ����ڿ̶��ߣ���������������Һ�����ʵ���Ũ�Ȳ��䣬��A����
B������ʱ��������ƿ�Ŀ̶��ߣ���Һ�����ƫС������c=
��֪��Һ�����ʵ���Ũ��ƫ��B��ȷ��
C������Һת������ƿ��û��ϴ���ձ��Ͳ����������ʵ����ʵ���ƫС������c=
��֪��Һ�����ʵ���Ũ��ƫС����C����
D�������ˮ�����˿̶��ߣ�ȡ������ˮʹҺ��ǡ�õ��̶��ߣ����ʵ����ʵ���ƫС������c=
��֪��Һ�����ʵ���Ũ��ƫС����D����
��ѡ��B��
��5����Ҫ̼���Ƶ�����m=0.25L��1mol?L-1��106g/mol=26.5g��
��5mol/L��̼������Һ�����ΪxmL����xmL��5mol/L=250mL��1mol/L����ã�x=50.0��
����Ӧ��ȡ5mol/L��̼������Һ�������50.0mL��
�ʴ�Ϊ��26.5g��50.0mL��
�ʴ�Ϊ���٢ۢݣ�
��2��ʵ������������������ƽ���ձ�����������250mL����ƿ����ͷ�ιܡ�ҩ�ף������������⣬��ȱ����������Ʒ��250mL����ƿ ��ͷ�ιܣ�
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��3�����������У����㡢�������ܽ⡢��ȴ����Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ȷ�IJ���˳��Ӧ��BEGACADF��
�ʴ�Ϊ��GACA��
��4��A������ҡ�Ⱥ���Һ����ڿ̶��ߣ���������������Һ�����ʵ���Ũ�Ȳ��䣬��A����
B������ʱ��������ƿ�Ŀ̶��ߣ���Һ�����ƫС������c=
| n |
| V |
C������Һת������ƿ��û��ϴ���ձ��Ͳ����������ʵ����ʵ���ƫС������c=
| n |
| V |
D�������ˮ�����˿̶��ߣ�ȡ������ˮʹҺ��ǡ�õ��̶��ߣ����ʵ����ʵ���ƫС������c=
| n |
| V |
��ѡ��B��
��5����Ҫ̼���Ƶ�����m=0.25L��1mol?L-1��106g/mol=26.5g��
��5mol/L��̼������Һ�����ΪxmL����xmL��5mol/L=250mL��1mol/L����ã�x=50.0��
����Ӧ��ȡ5mol/L��̼������Һ�������50.0mL��
�ʴ�Ϊ��26.5g��50.0mL��

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д�
�����Ŀ