��Ŀ����
������������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ���ᣬ����0.2mol/L��������Һ500mL���Իش��������⣺
��1��������Ҫ��ȡ��Ũ��������Ϊ______mL��
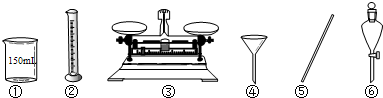
��2������ͼ�������⣬��ʵ�黹��Ҫ�õ��IJ��������У���ͷ�ιܡ�______��______����ͼ��ijͬѧת����Һ��ʾ��ͼ�������ͼ�еĴ���______��
��3�����ƹ����У����Ũ��ƫ�ߵIJ���������______������ţ���
A������ƿ������ˮϴ��δ����
B�����ձ����ܽ�����Һ����ת��������ƿ��Ȼ���ټ�����ˮ���̶���
C��δ��ˮϴ���ܽ��Ũ������ձ�
D������Ͳ��ȡŨ����ʱ�����Ӷ���
E������ʱ������Һ���ˮ���̶���
F��������ƿ��ת��Ũ��Һʱ������Һ�ε�������ƿ���森
��1��������Ҫ��ȡ��Ũ��������Ϊ______mL��
��2������ͼ�������⣬��ʵ�黹��Ҫ�õ��IJ��������У���ͷ�ιܡ�______��______����ͼ��ijͬѧת����Һ��ʾ��ͼ�������ͼ�еĴ���______��
��3�����ƹ����У����Ũ��ƫ�ߵIJ���������______������ţ���
A������ƿ������ˮϴ��δ����
B�����ձ����ܽ�����Һ����ת��������ƿ��Ȼ���ټ�����ˮ���̶���
C��δ��ˮϴ���ܽ��Ũ������ձ�
D������Ͳ��ȡŨ����ʱ�����Ӷ���
E������ʱ������Һ���ˮ���̶���
F��������ƿ��ת��Ũ��Һʱ������Һ�ε�������ƿ���森

��1����������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������ʵ���Ũ��Ϊ��
mol/L=18.4mol/L������0.2mol/L��������Һ500mL����ҪŨ��������Ϊ��
��0.0054L=5.4mL��
�ʴ�Ϊ��5.4��
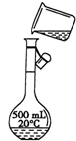
��2������Ͳ��ȡŨ���ᣬ���ձ����ܽ⣬�ò��������裬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�������Ҫ������Ϊ��10mL��Ͳ����ͷ�ιܡ��ձ�����������500mL����ƿ��ͼ��û��ʹ�ò�������������������Һ��������ƿ���棬
�ʴ�Ϊ��10mL��Ͳ����������Ӧ�ò�����������
��3��A������ƿ������ˮϴ��δ������ڶ���ʱ����Ҫ��������ˮ������ƿ��������ˮ��Ӱ�����ƽ������A����
B�����ձ����ܽ�����Һ����ת��������ƿ��Ȼ���ټ�����ˮ���̶��ߣ��ȵ���Һ���ƫ����ȴ����Һ�����С������c=
��֪�����Ƶ���ҺŨ��ƫ�ߣ���B��ȷ��
C��δ��ˮϴ���ܽ��Ũ������ձ����������Ƶ���Һ�����ʵ����ʵ���ƫС������c=
��֪�����Ƶ���ҺŨ��ƫ�ͣ���C����
D������Ͳ��ȡŨ����ʱ�����Ӷ�����������ȡ��Ũ�������ƫС������c=
��֪�����Ƶ���ҺŨ��ƫ�ͣ���D����
E������ʱ������Һ���ˮ���̶��ߣ����¼��������ˮ���ƫС������c=
��֪�����Ƶ���ҺŨ��ƫ�ߣ���E��ȷ��
F��������ƿ��ת��Ũ��Һʱ������Һ�ε�������ƿ���棬�������Ƶ���Һ�����ʵ����ʵ���ƫС������c=
��֪�����Ƶ���ҺŨ��ƫ�ͣ���F����
��ѡBE��
| 1000��1.84��98% |
| 98 |
| 0.2mol/L��0.5L |
| 18.4mol/L |
�ʴ�Ϊ��5.4��
��2������Ͳ��ȡŨ���ᣬ���ձ����ܽ⣬�ò��������裬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�������Ҫ������Ϊ��10mL��Ͳ����ͷ�ιܡ��ձ�����������500mL����ƿ��ͼ��û��ʹ�ò�������������������Һ��������ƿ���棬
�ʴ�Ϊ��10mL��Ͳ����������Ӧ�ò�����������
��3��A������ƿ������ˮϴ��δ������ڶ���ʱ����Ҫ��������ˮ������ƿ��������ˮ��Ӱ�����ƽ������A����
B�����ձ����ܽ�����Һ����ת��������ƿ��Ȼ���ټ�����ˮ���̶��ߣ��ȵ���Һ���ƫ����ȴ����Һ�����С������c=
| n |
| V |
C��δ��ˮϴ���ܽ��Ũ������ձ����������Ƶ���Һ�����ʵ����ʵ���ƫС������c=
| n |
| V |
D������Ͳ��ȡŨ����ʱ�����Ӷ�����������ȡ��Ũ�������ƫС������c=
| n |
| V |
E������ʱ������Һ���ˮ���̶��ߣ����¼��������ˮ���ƫС������c=
| n |
| V |
F��������ƿ��ת��Ũ��Һʱ������Һ�ε�������ƿ���棬�������Ƶ���Һ�����ʵ����ʵ���ƫС������c=
| n |
| V |
��ѡBE��

��ϰ��ϵ�д�
�����Ŀ