��Ŀ����
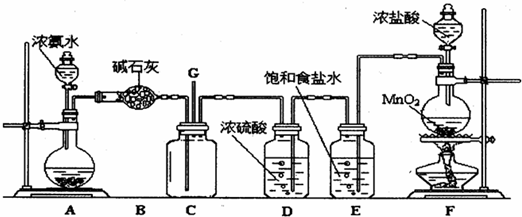
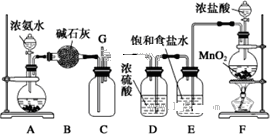
ijѧ����������װ��̽�������백��֮��ķ�Ӧ������CΪ��������������백����Ӧ��װ�á�
��ش��������⣺
��1��װ��A�е���ƿ�ڹ��岻��ѡ��
A����ʯ�� B����ʯ�� C������������ D���ռ�
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
��3��Bװ�õ����� ��Eװ�õ�����
��4��װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ��д����Ӧ�Ļ�ѧ����ʽ��
��5����װ��C��G���ݳ���β���п��ܺ��л���ɫ���ж����壬��Ҫ������
д�����ӷ���ʽ
ʵ�����Ʊ����������з���������ѡ�õ��� ��
�� ��̬�Ȼ�識��ȷֽ� �� Ũ��ˮ�м��������������
�� ����Ũ��ˮ �� ��̬�Ȼ�����������ƻ�ϼ���
��1��C
��2��NH3��H2O=NH3�� +H2O
��3���������壬����HCl
��4��8NH3 +3Cl2=N2 +6 NH4Cl
��5��Cl2 +2OH-=Cl- +ClO- +H2O
�ڢۢ�
����:

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�