��Ŀ����
A��B��C��D���ֶ�����Ԫ�ص�ԭ��������������A��Dͬ���壬B��Cͬ���ڡ�A��B��ɵĻ������Ϊ��̬������A��Bԭ����֮��Ϊ4��1����A��C��ɵ����ֻ������Һͱ���ΪҺ̬������A��Cԭ����֮��Ϊ1��1,����2��1����D��C��ɵ����ֻ����ﶡ���춼Ϊ��̬������D��Cԭ����֮��Ϊ1��1,����Ϊ2��1��
��1��д����ѧʽ����______________����_____________����_____________����_____________����_____________��
��2����������Էֱ����������ѧ��Ӧ����д��������Ӧ�����ӷ���ʽ��
______________________________________________________________________________________________________________________________________________________________
��3��A��C��Ԫ�صĵ��ʿ�����A��C��D����Ԫ���γɵĻ������ˮ��Һ����ȼ�ϵ�أ���д���缫��Ӧʽ��
������_________________________________________________________________________��
������________________________________________________________________________��
(1)CH4 H2O2 H2O Na2O2 Na2O
(2)2Na2O2+2H2O====4Na++4OH-+O2��
Na2O+H2O====2Na++2OH-
(3)2H2+4OH--4e-====4H2O
O2+2H2O+4e-====4OH-
����:
������,���������Һ̬����Ҫ��ˮ��������,��AΪHԪ��,CΪOԪ�ء�A��B�����γ�ԭ����֮��Ϊ4��1���⻯��,BΪ-4��,BΪ̼Ԫ��.���D����Ԫ���γɵĻ������е�ԭ�Ӹ����ȿ����ж�:DΪNaԪ��.

| A��X��Y��Z���ַ��ӵ��ȶ������� | B����������Ԫ�ع����γ����ֵ��� | C��X��Y��Z���ַ��ӵķе������� | D��X��Y��Z���ַ��Ӿ�Ϊ���Է��� |

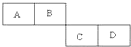 A��B��C��D���ֶ�����Ԫ�������ڱ��е����λ�������ʾ��A�ĵ�����ˮ������Ӧ������ȡˮú����
A��B��C��D���ֶ�����Ԫ�������ڱ��е����λ�������ʾ��A�ĵ�����ˮ������Ӧ������ȡˮú����