��Ŀ����
����Ŀ����У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ36.5%��Ũ�����ܶ�Ϊ119 g/cm3���Ƴ�240mL0.1mol��L��1��������Һ��
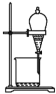
(1)��ͼ��ʾ�����У�����������Һ����Ҫ����_________(��ͼ��Ӧ���������)�� ��ͼ�����������⣬����������Һ����Ҫ�IJ���������_________�������ð�ʹ�õ��Ⱥ�˳��ֱ���_________��
(2)���ݼ��㣬��Ҫ��ȡ_______mLŨ���ᣬ�������̻����У���ʵ����ͲӦʹ�õ���_______������ƿӦʹ��_______��(�ں�������д��Ӧ����ţ������֣���ͬ)
A��10mL B��100mL C��250mL D��500mL
(3)��������ʱ������������ȷ��ֻ��������ijһ���������ʹ������Һ�����ʵ���Ũ��ƫ�ߵ���______��ƫ�͵���_______��(�����)
������ƿ������������ˮ ���ձ��Ͳ���û��ϴ��2-3�� ��ϡ��ŨHClʱ��û����ȴ������ת�Ƶ�����ƿ�� ����ȡŨ����ʱ���� �����Ƶ���Һװ��ྻ�ĵ�����������ˮ���Լ�ƿ�� ����ʱ����
���𰸡�C ������ ���������� 2.1 A C �ۢܢ� �ڢ�
��������
(1)��������һ�����ʵ���Ũ�ȵ���Һʹ�õ�����������Ҫ��������ȱ�ٵ��������ٸ��ݲ������ڲ����е����ý��
(2)����c=![]() �������ҪŨ�����Ũ�ȣ��ٸ�������250mL 0.1molL-1��������Һ��Ҫ���Ȼ�������ʵ����������Ҫ�����������240mL��Һ��Ҫ250mL����ƿ��
�������ҪŨ�����Ũ�ȣ��ٸ�������250mL 0.1molL-1��������Һ��Ҫ���Ȼ�������ʵ����������Ҫ�����������240mL��Һ��Ҫ250mL����ƿ��
(3)����c=![]() ����������ҺŨ�ȵı仯��
����������ҺŨ�ȵı仯��
(1)��Һ©��������ȡ�ͷ�Һ������һ�����ʵ���Ũ����Һ���÷�Һ©������ѡC����ȱ�ٲ���������Ũ����ϡ��ʱ�ò��������裬ת��Һ��ʱ�ò�����������
(2)��������Ϊ37%��Ũ����(�ܶ�Ϊ1.19g/cm3)�����ʵ���Ũ��Ϊ��c=![]() =
=![]() =11.9mol/L������240mL��Һ��Ӧѡ��250mL������ƿ������Ũ�������V=
=11.9mol/L������240mL��Һ��Ӧѡ��250mL������ƿ������Ũ�������V=![]() =0.0021L=2.1mL����ѡ��10mL��Ͳ��
=0.0021L=2.1mL����ѡ��10mL��Ͳ��
(3)��������ƿ������������ˮ����Ϊ���ƹ�����Ҫ��ˮ���ݣ�����ƿ��ԭ������ˮ��������յ���Һ������Ӱ�죬��˲������������Һ��Ũ����Ӱ�죻
���ձ��Ͳ���û��ϴ��23�Σ�����ϴ��Һת������ƿ�У������������ʧ������c=![]() �����ʵ����ʵ���nֵƫС�����յ���ҺŨ��ƫ�ͣ�
�����ʵ����ʵ���nֵƫС�����յ���ҺŨ��ƫ�ͣ�
��ϡ��Ũ����ʱ��û����ȴ������ת�Ƶ�����ƿ�У���Һ��ȴ������������Һ���ƫС�� ����c=![]() ��ʽ�е�V��ҺƫС�����յ���ҺŨ��ƫ�ߣ�
��ʽ�е�V��ҺƫС�����յ���ҺŨ��ƫ�ߣ�
����ȡŨ����ʱ���ӣ�������ȡ��Ũ��������ƫ�������ʵ����ʵ���ƫ����c=![]() ��ʽ�����ʵ����ʵ���nֵƫ�����յ���ҺŨ��ƫ�ߣ�
��ʽ�����ʵ����ʵ���nֵƫ�����յ���ҺŨ��ƫ�ߣ�
�����Ƶ���Һװ��ྻ�ĵ�����������ˮ���Լ�ƿ�У��൱��ϡ�������ƺõ���Һ���������Һ��Ũ��ƫ�ͣ�
��������ʱ���ӣ��������Һ�����ƫС������c=![]() ��ʽ�е�V��ҺƫС����Һ��Ũ��ƫ�ߣ�
��ʽ�е�V��ҺƫС����Һ��Ũ��ƫ�ߣ�
��ʹ������Һ�����ʵ���Ũ��ƫ�ߵ��ǣ��ۢܢޣ�ʹ������Һ�����ʵ���Ũ��ƫ�͵��ǣ��ڢݡ�

����Ŀ��ijС�����ʵ�����ȼ�յIJ��P�����ʽ�����֤��ʵ��װ������ͼ��ʾ��
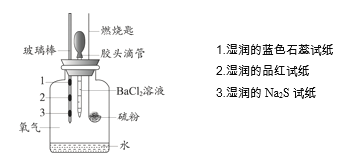
����ʵ����ʵ�����ý����������
ʵ����ʵ | ���ý��� | |
A | ʪ���Ʒ����ֽ��ɫ | ֤����ȼ�յIJ�������SO2 |
B | ʪ���Na2S��ֽ�ϳ��ֵ���ɫ���� | ֤�����ȼ�ղ����ܱ���ԭ���� |
C | ʪ�����ɫʯ����ֽ��� | ֤�����ȼ�ղ��������������� |
D | ����BaCl2��Һ������ɫ���� | ֤����ȼ�յIJ�������SO3 |
A.AB.BC.CD.D