��Ŀ����
17��ȼ�շ��Dzⶨ�л����������ʽ��һ����Ҫ��������ش��������⣺��1������4���л����1mol���ֱ��������������г��ȼ�գ���������������C������ţ�
A��CH4 B��C2H4 C��C2H6 D��C2H6O
��2��0.1molij��B��ȫȼ�պ����ɵ�CO2�������Ϊ6.72L��STP�������ɵ�ˮΪ7.2g����0.1mol B�У���̼������Ϊ3.6g�����������Ϊ0.8g��B�ķ���ʽΪC3H8��
���� ��1�������л���ȼ�յ�ͨʽ���㣬ͨʽΪ��CxHyOz+��x+$\frac{y}{4}$-$\frac{z}{2}$��O2��xCO2+$\frac{y}{2}$H2O��ȷ�������ʵ��������������ʵ�����
��2������CO2�����������Cԭ�ӵ����ʵ���������ˮ����������Hԭ�ӵ����ʵ��������������������ʵ����ɼ������ķ���ʽ���Ӷ���ȷ���ṹ��ʽ��
��� �⣺��1��A��1molCH4����Ϊ2mol������B��1molC2H4������Ϊ3mol������C��1molC2H6������Ϊ3.5mol������ D��1molC2H6O������Ϊ3mol������������������������C2H6����ѡC��
��2�����ɵ�CO2�������Ϊ6.72L��STP����0.3mol������n��C��=n��CO2��=0.3mol������m��C��=12��0.3=3.6g��
���ɵ�ˮΪ7.2g����0.4mol������n��H��=2n��H2O��=0.8mol����m��H��=0.8g��n��������n��C����n��H��=0.1��0.3��0.8=1��3��8��������B�ķ���ʽΪ��C3H8���ʴ�Ϊ��3.6��0.8��C3H8��
���� ���⿼�����ķ���ʽ��ȷ������Ŀ�ѶȲ�����ע������1mol���к���C��Hԭ�ӵ����ʵ����ɼ������ķ���ʽ��

 ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д� ����5��2���ϵ�д�
����5��2���ϵ�д�| A�� | Cl2 | B�� | NO2 | C�� | SO2 | D�� | H2O2 |
| A�� | ��֬����ֵ��ߵ�Ӫ������ | |
| B�� | �����Ҵ����ܷ���������Ӧ | |
| C�� | ����ϩ��ʹ���Ը��������Һ��ɫ | |
| D�� | ��Na2CO3��Һ��������CH3COOH��CH3COOCH2CH3 |
| A�� | �ü��������ڹ۲�һЩ�������ӵ��˶� | |
| B�� | �ü��������ڹ۲�һЩ����ӵ����ʵĺϳ� | |
| C�� | �ü���ʹ�۲�ˮ�ķ����Ŵؽṹ��Ϊ���� | |
| D�� | �ü��������ڷֱ�ӫ������и��ֲ�ͬ�����ԭ�� |
| ѡ�� | �� | �� | �� | ʵ����� | 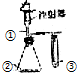 |
| A | ϡ���� | Na2SO4 | ��ˮ | SO2����Ư���� | |
| B | Ũ��ˮ | CaO | AlCl3��Һ | ��ɫ�������ɺ����ܽ� | |
| C | ˫��ˮ | MnO2 | KI��Һ | �����ԣ�O2��I2 | |
| D | ϡ���� | Na2CO3 | Na2SiO3��Һ | ���ԣ����̼����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | NO2--��ɫ���� | B�� | BaSO4--����ɫ���� | ||
| C�� | Al��OH��3--��ɫ���� | D�� | H2O--��ɫҺ�� |
 6�����ڶ���������Ԫ�ص����λ�������MԪ��ԭ�Ӻ����������YԪ��ԭ�Ӻ����������2����WԪ��ԭ�ӵĴ�����������������������2�����û�ѧ����ش��������⣺
6�����ڶ���������Ԫ�ص����λ�������MԪ��ԭ�Ӻ����������YԪ��ԭ�Ӻ����������2����WԪ��ԭ�ӵĴ�����������������������2�����û�ѧ����ش��������⣺| X | Y | Z | |
| W | M | Q |
��2��X��Y��Z����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳����r��N����r��O����r��F����
��3��W��M��Q����Ԫ������������Ӧ��ˮ�����У�������ǿ������˳����HClO4��H2SO4��H2SiO3��
��4��X�ĺ�10�����ӵ���̬�⻯�����һ����Ҫ�Ļ���ԭ�ϣ�������X������������Ӧ��ˮ������Һ��Ӧ�������ң�����Һ�е�����Ũ���ɴ�С��˳����c��NO3-����c��NH4+����c��H+����c��OH-����X�ĺ�18�����ӵ���̬�⻯��������������һ�ֻ����ͼ���ȼ�ϵ�أ���ع���ʱ�������ĵ缫��Ӧʽ��N2H4-4e-+4OH-�TN2+4H2O��
��5����ҵ������ͼ��ʾװ�õ��QԪ�ص�������Һ�������һ�ֺ�QԪ�ص������������÷�Ӧ�����ӷ���ʽ��Cl-+H2O $\frac{\underline{\;���\;}}{\;}$ClO-+H2�������ĵ���ʽ
 ��
����6����������Ҫ�ɷ���HCl�����й���±��������ʱȽ��д������AC
A�����ԣ�HF��HCl��HBr��HI B�����ܣ�H-F��H-Cl��H-Br��H-I
C���е㣺HF��HCl��HBr��HI D����ԭ�ԣ�HF��HCl��HBr��HI��
 ����Ӧ2Fe3++Cu�T2Fe2++Cu2+��Ƴ���ͼ��ʾԭ��أ��ɹ�ѡ��ĵ������Һ��FeCl3��CuSO4������Һ��
����Ӧ2Fe3++Cu�T2Fe2++Cu2+��Ƴ���ͼ��ʾԭ��أ��ɹ�ѡ��ĵ������Һ��FeCl3��CuSO4������Һ��