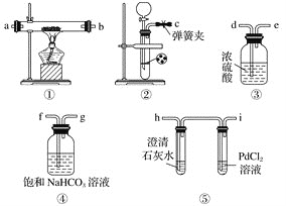
��Ŀ����
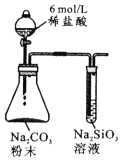
����Ŀ��������(FeCr2O4)�к���Al2O3��Fe2O3�����ʣ��Ը�����Ϊԭ���Ʊ��ظ����(K2Cr2O7)�Ĺ�������ʾ��ͼ����(���ֲ�����������)��
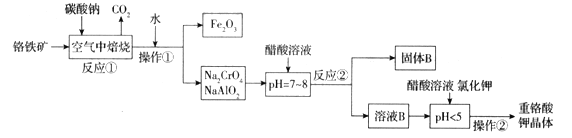
��1��������(FeCr2O4)��дΪ���������ʽΪ___________________��
��2����Ӧ���з�����������Ҫ��Ӧ������һ����Ҫ��Ӧ�Ļ�ѧ����ʽΪ4FeCr2O4+8Na2CO3+7O2![]() 8Na2CrO4+2Fe2O3+8CO2����һ����Ҫ��Ӧ�Ļ�ѧ����ʽΪ ______________________________��
8Na2CrO4+2Fe2O3+8CO2����һ����Ҫ��Ӧ�Ļ�ѧ����ʽΪ ______________________________��
��3��д����Ӧ�������ɹ���B�����ӷ���ʽΪ_________________________________��
��4��pH < 5ʱ��������Ӧ��____________________________________��д���ӷ���ʽ����Na2Cr2O7 + 2KCl = K2Cr2O7��+ 2NaCl��
�±���������ʵ��ܽ�����ݣ�
���� | �ܽ�� ( g / 100 g H2O ) | ||
0�� | 40�� | 80�� | |
KCl | 28 | 40.1 | 51.3 |
NaCl | 35.7 | 36.4 | 38 |
K2Cr2O7 | 4.7 | 26.3 | 73 |
Na2Cr2O7 | 163 | 215 | 376 |
���K2Cr2O7����IJ����ɶಽ��ɣ������Ǽ���KCl���塢����Ũ����________________��_____________��ϴ�ӡ�����õ����塣
��5��ij�־ƾ��������У�K2Cr2O7�����������½��Ҵ�����Ϊ���ᣬ��������ԭΪ���۸����ӣ��÷�Ӧ���������뻹ԭ�������ʵ�����Ϊ_________________��
��6����������(��ˮ)�������ŷŶԻ����м���Σ������ⷨ�Ǵ�������Ⱦ��һ�ַ��������ʱ������������Fe(OH)3��Cr(OH)3��������(��֪Ksp[Fe(OH)3] = 4.0��10-38��KspCr(OH)3] = 6.0��10-31)����֪�������Һ��c(Cr3+)Ϊ3.0��10-5mol/L������Һ��c(Fe3+)Ϊ________________ mol/L��
���𰸡�FeO��Cr2O3 Na2CO3+ Al2O3 ![]() 2NaAlO2 + CO2�� AlO2�� + CH3COOH + H2O = Al(OH)3��+ CH3COO�� 2CrO42-+2H+
2NaAlO2 + CO2�� AlO2�� + CH3COOH + H2O = Al(OH)3��+ CH3COO�� 2CrO42-+2H+ ![]() Cr2O72-+H2O ��ȴ�ᾧ ���� 2: 3 2.0��10��12
Cr2O72-+H2O ��ȴ�ᾧ ���� 2: 3 2.0��10��12
��������
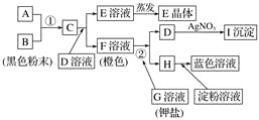
��1������FeCr2O4�и�Ԫ�صĻ��ϼۣ���д���ɣ�
��2��������ͼ���Կ�������Ӧ���л��и������г�ȥ���ʣ�Al2O3���ķ�Ӧ����̼���ƺ���������Ӧ��
��3����Ӧ����pH![]() 5�Ĵ�����Һ�У����ɳ�����
5�Ĵ�����Һ�У����ɳ�����
��4��������������CrO42-ת����Cr2O72-��Ҫ��þ���IJ���Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��5��![]() �����������½��Ҵ�����Ϊ���ᣬ��������ԭΪ���۸����ӣ����ݵ���ת���غ㣬�����ó���������ԭ�������ʵ���֮�ȣ�
�����������½��Ҵ�����Ϊ���ᣬ��������ԭΪ���۸����ӣ����ݵ���ת���غ㣬�����ó���������ԭ�������ʵ���֮�ȣ�
��6�������������Ksp[Cr(OH)3] = 6.0��10-31)��Ksp[Fe(OH)3] = 4.0��10-38���������㣻
��1������������Ԫ��Ϊ+2�ۣ���Ԫ��Ϊ+3�ۣ�����![]() ��дΪ���������ʽΪ
��дΪ���������ʽΪ![]() ��
��
�������![]() ��
��
��2��������ͼ���Կ�������Ӧ���л��У��������г�ȥ���ʣ�Al2O3���ķ�Ӧ����Na2CO3+ Al2O3 ![]() 2NaAlO2 + CO2����
2NaAlO2 + CO2����
�������Na2CO3+ Al2O3 ![]() 2NaAlO2 + CO2����
2NaAlO2 + CO2����
��3����Ӧ����pH![]() 5�������£����ɳ���������BӦΪAl(OH)3���������ӷ���ʽΪAlO2- + CH3COOH + H2O = Al(OH)3��+ CH3COO����
5�������£����ɳ���������BӦΪAl(OH)3���������ӷ���ʽΪAlO2- + CH3COOH + H2O = Al(OH)3��+ CH3COO����
�������AlO2�� + CH3COOH + H2O = Al(OH)3��+ CH3COO����
��4��������������CrO42-ת����Cr2O72-�����ӷ���ʽΪ��2CrO42-+2H+ ![]() Cr2O72-+H2O ��Ҫ��þ���IJ���Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
Cr2O72-+H2O ��Ҫ��þ���IJ���Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
�����Ϊ��2CrO42-+2H+ ![]() Cr2O72-+H2O ����ȴ�ᾧ�����ˡ�
Cr2O72-+H2O ����ȴ�ᾧ�����ˡ�
��5��![]() �����������½��Ҵ�����Ϊ���ᣬ��������ԭΪ���۸����ӣ��÷�Ӧ�����ӷ���ʽΪ
�����������½��Ҵ�����Ϊ���ᣬ��������ԭΪ���۸����ӣ��÷�Ӧ�����ӷ���ʽΪ![]() �������������ͻ�ԭ�������ʵ���֮��Ϊ2:3��
�������������ͻ�ԭ�������ʵ���֮��Ϊ2:3��
�������2:3.
��6���������Һ��![]() Ϊ
Ϊ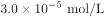 ��������Һ��
��������Һ��![]() ����
����![]() ������
������![]()
![]()
![]() ��
��
�������![]() ��
��

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
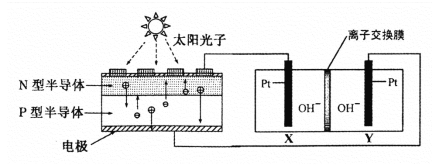
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�����Ŀ����ͼ��ʾ��x��y�ֱ���ֱ����Դ��������ͨ�����a����������������b�����崦����ɫ��ζ������ų���������һ�������(����)
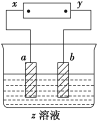
ѡ�� | a���� | b���� | x�缫 | z��Һ |
A | п | ʯī | ���� | ����ͭ |
B | ʯī | ʯī | ���� | �������� |
C | �� | �� | ���� | ������ |
D | ͭ | ʯī | ���� | �Ȼ�ͭ |
A. A B. B C. C D. D
����Ŀ��������ʵ������һ���ܵó���Ӧ���۵���
ѡ�� | A | B | C | D |
װ��ͼ |
|
|
|
|
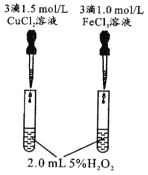
���� | �ұ��Թܲ������ݽϿ� | ���������ػ�ɫ���ұ��������ɫ | �Թ����ȳ��ֵ���ɫ���壬����ֻ�ɫ���� | �Թ���Һ������ |
���� | �����ԣ�Fe3+��Cu2+ | �����ԣ�Br2��I2 | Ksp��AgCl��AgBr��AgI | �ǽ����ԣ�C��Si |
A. AB. BC. CD. D