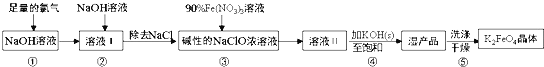
��Ŀ����
����Ŀ�������е⺬���Ƚϸߣ��Ӻ�����ȡ��IJ�������:
(1)���ɺ����������յ�������________���������к��н϶�KI��������������ˮ��Ȼ����˵õ�������Һ��
(2)��������Һ�м��������H2O2�����Һ���õ��غ�ɫ���е��ʵ��ˮ��Һ����д�����ӷ�Ӧ����ʽ:________________________________��
(3)���������ˮ��Һ�м�������CCl4�������ã���I2��ת�뵽CCl4���У�������̽�_____________________������Ϊ____________________________��
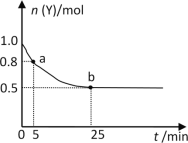
(4)3I2��6KOH=5KI��KIO3��3H2O��1.5mol I2��ȫ��Ӧת�Ƶ��ӵ����ʵ���Ϊ_____mol���������뻹ԭ�������ʵ���֮��Ϊ___________��
(5)ijһ��Ӧ��ϵ�з�Ӧ��������ﹲ6������:O2��K2Cr2O7��Cr2��SO4��3��H2SO4��H2O��H2O2����֪�÷�Ӧ��H2O2ֻ�������¹���:H2O2��O2
�ٸ÷�Ӧ�У�������Ϊ____________����������Ϊ____________��
��д���÷�Ӧ�Ļ�ѧ����ʽ__________________________��
���õ����ŷ���ʾ������Ӧ�е���ת�Ʒ������Ŀ________________��
���𰸡����� H2O2+2I-+2H+=2H2O+I2 ��ȡ ����������Һ�ֿ����ϲ�Ϊ��ɫ���²�Ϊ�Ϻ�ɫ 2.5mol 5:1 K2Cr2O7 O2 K2Cr2O7+3H2O2+4H2SO4=Cr2(SO4)3+7H2O+3O2��+K2SO4 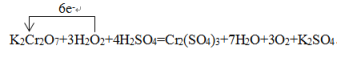
��������
��1��������������������
��2������������ԭ��Ӧ������д��
��3����ȡ���������Ȼ�̼���ܶȱ�ˮ���л���λ���²㣻
��4������������ԭ��Ӧ�����غ���м��㣻
��5������������ԭ��Ӧԭ������жϣ�������ָ��õ����ӵķ�Ӧ��һ����
(1)���ɺ����������յ��������������������к��н϶�KI��������������ˮ��Ȼ����˵õ�������Һ��
(2)��������Һ�к��е⻯�أ����������H2O2�����Һ�������������ǿ�����ԣ��õ��غ�ɫ���е��ʵ��ˮ��Һ�����ӷ�Ӧ����ʽH2O2+2I-+2H+=2H2O+I2��
(3)���������ˮ��Һ�м�������CCl4�������ã���I2��ת�뵽CCl4���У�������̽���ȡ������Ϊ����������Һ�ֿ����ϲ�Ϊ��ɫ���²�Ϊ�Ϻ�ɫ��
(4)3I2��6KOH=5KI��KIO3��3H2O���ⵥ�ʷ����绯��Ӧ��3mol�ⵥ������5mol��ԭ����⻯�أ�1mol�����������أ�ת�Ƶ�����Ϊ5mol����1.5mol I2��ȫ��Ӧת�Ƶ��ӵ����ʵ���Ϊ2.5mol������ϵ��֮�ȵ������ʵ���֮�ȣ�������������KIO3Ϊ1mol����ԭ��I2Ϊ0.5mol����ԭ����KIΪ5mol����������I2Ϊ2.5mol�����Ը÷�Ӧ�������뻹ԭ�������ʵ���֮��Ϊ5��1��
(5)ijһ��Ӧ��ϵ�з�Ӧ��������ﹲ6������:O2��K2Cr2O7��Cr2��SO4��3��H2SO4��H2O��H2O2����֪�÷�Ӧ��H2O2ֻ�������¹���:H2O2��O2����Ԫ�ػ��ϼ����ߣ�������������ԭ��������������ԭ��Ӧ���ٽ�ԭ��ͻ��ϼ�����ԭ����ʽ����ΪK2Cr2O7+3H2O2+4H2SO4=Cr2(SO4)3+7H2O+3O2��+K2SO4��
�ٸ÷�Ӧ�У�������ΪK2Cr2O7����������ΪO2��
���õ����ŷ���ʾ������Ӧ�е���ת�Ʒ������Ŀ��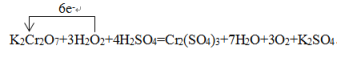 ��
��