��Ŀ����
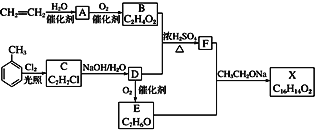
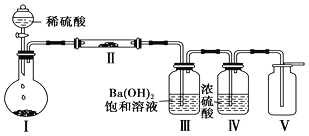
����Ŀ����ͼ����ʵ���ҽ��ж��������Ʊ�������ʵ������װ�ã����̶ֹ�װ��δ������
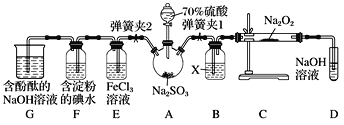
(1)����װ��װ�ú���Ҫ����A��Dװ�õ������ԣ������������__________��Ȼ����D��װ��ˮ��Ȼ����A���۲쵽D��������ð�����ƿ��ƾ��ƻ��ɿ�˫�֣�D�е�����ˮ���γ��Ҹ߶ȱ��ֲ��䣬˵��װ�����������á�
(2)װ��D��ʢ��NaOH��Һ��������___________��
(3)�رյ��ɼ�1���ɼ�2�������������E��F��G�У���˵��I����ԭ������SO2������Ϊ_____��������Ӧ�����ӷ���ʽ��________________��
(4)Ϊ����֤E��SO2��FeCl3������������ԭ��Ӧ�����������ʵ�飺ȡE�е���Һ������Һ�м�����ϡ�����ữ��BaCl2��Һ��������ɫ������˵��SO2��FeCl3������������ԭ��Ӧ��
���������Ƿ������________(����������������������)��ԭ����_____________��
(5)ʵ�������G�к���̪��NaOH��Һ����ɫ����ʵ��֤��SO2����Ư���Ի���������ˮ�����ԣ������ʵ����֤��______________________��
���𰸡��رյ��ɼ�2�ͷ�Һ©�����������ɼ�1 ����δ��Ӧ��SO2����ֹ��Ⱦ���� F����Һ��ɫ��ȥ SO2��I2��2H2O===2I����SO42-��4H�� ������ E���ܽ��SO2��ϡ���ᷴӦҲ����SO42- ȡ��ɫ�����Һ���μ�NaOH��Һ������죬��֤����ɫ����SO2����ˮ������(��ȡ��ɫ�����Һ�����ȣ���δ���ɫ����֤��SO2����ˮ������)
��������
��1������װ�õ�������ʱ����Ҫ�γ��ܷ�ϵͳ��
��2��SO2���ж�������������ܱ�NaOH��Һ���ա�
��3��I����ԭ������SO2������I2��SO2�ķ�Ӧ��ɵó�ʵ����������ӷ���ʽ��
��4������ʵ�����ʱ������Ҫ��������������ԭ��Һ�����ӵķ�Ӧ�Ƿ���ʵ����ɸ��š�
��5��ȡ��ɫ�����Һ���μ�NaOH��Һ������죬��֤����ɫ����SO2����ˮ������(��ȡ��ɫ�����Һ�����ȣ���δ���ɫ����֤��SO2����ˮ������)��
��1������װ�õ�������ʱ����Ҫ�γ��ܷ�ϵͳ�����Թرյ��ɼ�2�ͷ�Һ©�����������ɼ�1���ʴ�Ϊ���رյ��ɼ�2�ͷ�Һ©�����������ɼ�1��
��2��SO2���ж�������������ܱ�NaOH��Һ���գ��ʴ�Ϊ������δ��Ӧ��SO2����ֹ��Ⱦ������
��3����ˮ������SO2����������ԭ��Ӧ���������������ᣬ���ӷ���ʽΪ��SO2��I2��2H2O===2I����SO42-��4H������Ϊ��ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�ԣ�����I����ԭ������SO2��F�еⵥ�ʱ�ɵ������ˣ�����Һ��ɫ��ȥ���ʴ�Ϊ��F����Һ��ɫ��ȥ��SO2��I2��2H2O===2I����SO42-��4H����
��4������������Ϊϡ�������ǿ�����ԣ��ܰѶ�������������SO42-���ʴ�Ϊ����������E���ܽ��SO2��ϡ���ᷴӦҲ����SO42-��
��5��ʵ�������G�к���̪��NaOH��Һ����ɫ��ȡ��ɫ�����Һ���μ�NaOH��Һ������죬��֤����ɫ����SO2����ˮ������(��ȡ��ɫ�����Һ�����ȣ���δ���ɫ����֤��SO2����ˮ������)���ʴ�Ϊ��ȡ��ɫ�����Һ���μ�NaOH��Һ������죬��֤����ɫ����SO2����ˮ������(��ȡ��ɫ�����Һ�����ȣ���δ���ɫ����֤��SO2����ˮ������)��

 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�����Ŀ���������ӷ���ʽ����д�����۾���������( )
ѡ�� | ���ӷ���ʽ | ���� |
A | ��2 mol Cl2ͨ�뺬1 mol FeI2����Һ�У� 2Fe2����2I����2Cl2===2Fe3����4Cl����I2 | ��ȷ��Cl2�������ɽ�Fe2����I�������� |
B | Ba(HCO3)2��Һ��������NaOH��Һ��Ӧ�� Ba2����HCO3-��OH��===BaCO3����H2O | ��ȷ����ʽ����Ӧ�������κ�ˮ |
C | ����SO2ͨ��NaClO��Һ�У� SO2��H2O��ClO��===HClO��HSO3- | ��ȷ��˵�����ԣ�H2SO3ǿ��HClO |
D | 1 mol/L��NaAlO2��Һ��2.5 mol/L��HCl��Һ�������ϣ� 2AlO2-��5H��===Al3����Al(OH)3����H2O | ��ȷ����һ����Ӧ�͵ڶ�����Ӧ���ĵ�H�������ʵ���֮��Ϊ2��3 |
A. A B. B C. C D. D